About
Despite advances in detection and treatment, cancer still claims the lives of more than 600,000 people each year (equivalent to one person every minute) in this country [1]. The advent of cancer immunotherapy has recently revolutionized cancer treatment and provided reasons to be optimistic that this statistic will soon improve. For example, antibody-based immune checkpoint inhibitors (ICIs) against CTLA-4, PD-1, or PD-L1 can induce long-lasting clinical responses in patients who have advanced or metastatic cancers that were previously considered intractable. Other approaches to overcome tumor-mediated immune evasion and boost the anticancer immune response (such as vaccines, adoptive T cell therapies/ CAR-T, and oncolytic viruses) are either approved or showing promise in human clinical trials. Nonetheless, the available immuno-therapies can have significant side effects and are not effective for all types of cancer. Even for generally responsive cancer types (e.g. melanoma, lung, kidney, bladder, head-and-neck), ICI therapy often fails due to intrinsic or acquired resistance. Much more work is needed for cancer immunotherapy to reach its full potential of reliably attaining immune-mediated eradication of all types of malignancies with minimal systemic toxicity [2-4].
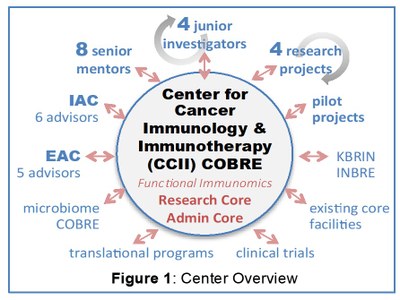 The overall goal of this project is to establish a Center of Biomedical Research Excellence (COBRE) program with a focus on understanding the interplay between cancer and the host immune system in order to identify new therapeutic targets and develop innovative treatment strategies. To achieve this goal, we plan to create the Center for Cancer Immunology and Immunotherapy (CCII) at the University of Louisville (Figure 1). Drs. Jun Yan and Jason Chesney will lead the CCII; these PIs are highly regarded experts in the field who have made seminal contributions spanning from basic cancer immunology to pivotal clinical trials of cancer immunotherapies. During the first 5-year period (Phase 1) of this COBRE, we propose four specific aims:
The overall goal of this project is to establish a Center of Biomedical Research Excellence (COBRE) program with a focus on understanding the interplay between cancer and the host immune system in order to identify new therapeutic targets and develop innovative treatment strategies. To achieve this goal, we plan to create the Center for Cancer Immunology and Immunotherapy (CCII) at the University of Louisville (Figure 1). Drs. Jun Yan and Jason Chesney will lead the CCII; these PIs are highly regarded experts in the field who have made seminal contributions spanning from basic cancer immunology to pivotal clinical trials of cancer immunotherapies. During the first 5-year period (Phase 1) of this COBRE, we propose four specific aims:
1. Establish the administrative and mentoring infrastructure for the CCII. The Administrative Core will oversee and coordinate all activities of the CII. It will provide infrastructure and personnel for selecting and mentoring junior investigators from any discipline who have research interests in the thematic area. We have already identified 8 senior investigators at our institution to serve as mentors; all are well established as experts in cancer immunology/immunotherapy. Additional senior investigators with expertise in other relevant areas are available to serve as mentors or on the Internal Advisory Committee (IAC). Five investigators who have international reputations in the thematic area will serve on the CCII External Advisory Committee (EAC).
2. Create a CCII research core that provides new capabilities while leveraging existing facilities. We will establish the Functional Immunomics Core (FI Core) to support research in the CCII focus area. Utilizing new equipment purchased with institutional funds (mass cytometry, cell sorting) and leveraging existing facilities (genomics, biorespository, tissue processing), the FI Core will provide molecular/cellular immune profiling for mouse and human samples, and will be used for all CCII research projects. CCII investigators will also have access to other COBRE/INBRE resources such as Functional Microbiomics and KBRIN Bioinformatics cores.
3. Support the research and career development of junior investigators in the thematic area. The CCII will provide financial support, core access, mentoring, and networking opportunities for promising investigators who are conducting innovative research in the thematic area but who have not yet received R01-type funding. We have selected 4 junior investigators (from 3 different departments) as the inaugural CCII mentees. These four are PIs for projects that are very diverse, yet share a common theme of defining the mechanisms by which cancers escape immune detection and evaluating ideas that could translate into new biomarkers or treatments.
4. Develop and initiate a plan for long-term sustainability and growth of the CCII. We will seek to grow the CCII by retaining and nurturing existing talent, by recruiting new investigators, and by creating additional cross-disciplinary collaborations that leverage institutional strengths. A robust pipeline of junior investigators to replace “graduating” project PIs and institutional support to provide a pilot project program are already in place.
We anticipate numerous positive outcomes by the end of the 5-year period (e.g. 4–8 junior PIs winning their first R01 grants, state-of-the-art facilities, increased funding for CCII senior faculty, high impact papers) that will establish the CCII as a leading center in the field and ultimately translate into saving lives.
 Facebook
Facebook Twitter
Twitter Linkedin
Linkedin