Behavioral, Cognitive, and Affective Responses to Lung Cancer Screening
Study Personnel
Principal Investigator: Jamie L. Studts, Ph.D.
Co-Principal Investigator: Renato LaRocca, M.D., Kentuckiana Cancer Institute (KCI)
Co-Investigator: A. Scott LaJoie, Ph.D.
Study Coordinators: Christopher N. Barnes and Christina R. Studts
Graduate Students: Joshua L. Ruberg, and Tiffany Cross
Study Synopsis:
This research project centers on understanding and modelling the behavioral, cognitive and affective responses of two Lung Cancer screening modalities, Chest X-ray (CXR) and low-dose Spiral Computed Tomography (SCT) under the protocol of a randomized clinical trial. The trial, the Jewish Hospital Early Detection Lung Cancer Screening Study (JH-EDLSS), was composed of three years of annual screening followed by two years of additional follow-up and was a joint project between Jewish Hospital and the University of Louisville/ Brown Cancer Center. The trial began in 1999 and is expected to complete follow-up data collection in December 2006.
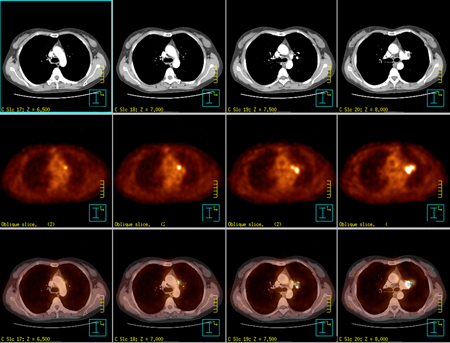 Specific Aim #1: The primary aim of this research is to compare longitudinal changes in behavioral, cognitive and affective responses to lung cancer screening. As SCT is a more sensitive screening modality, the research will explore whether participation in SCT leads to different behavioral, cognitive, and affective outcomes when compared to CXR.
Specific Aim #1: The primary aim of this research is to compare longitudinal changes in behavioral, cognitive and affective responses to lung cancer screening. As SCT is a more sensitive screening modality, the research will explore whether participation in SCT leads to different behavioral, cognitive, and affective outcomes when compared to CXR.
Hypothesis #1: Compared to CXR group participants, SCT group participants will report: (a) a greater decrease in smoking behavior; (b) a greater protocol non-adherence rate; (c) a greater readiness to quit smoking; (d) a greater increase in perceived threat of lung cancer; and (e) a greater increase in lung cancer worry.
Specific Aim #2: The secondary aim of this research is to identify predictors of longitudinal change in behavior following participation in lung cancer screening. The proposed research will test the ability of the Extended Parallel Processing Model to explain changes in smoking status and adherence among participants in a lung cancer screening trial. In conjunction with sociodemographic and clinical data, cognitive, and affective variables will be used to predict behavioral responses to lung cancer screening.
Hypothesis #2a: Sociodemographic, clinical, cognitive, and affective variables will be predictive of changes in smoking behavior among all study participants. Decreases in smoking behavior will be related to: (a) younger age; (b) receiving an abnormal screening result; (c) higher perceived threat of lung cancer; (d) greater readiness to quit smoking; (e) higher perceived smoking cessation efficacy; and (f) higher lung cancer worry.
Hypothesis #2b: Sociodemographic, clinical, cognitive, and affective variables will predict adherence to the lung cancer screening protocol. Non-adherence will be related to: (a) younger age; (b) receiving an abnormal screening result; (c) higher perceived threat of lung cancer; and (e) higher lung cancer worry.
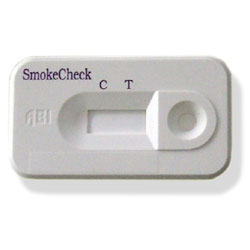 Specific Aim #3: The tertiary aim of this research is to examine concordance between self-report smoking behavior and a biochemical index of smoking behavior in a sub-sample of study participants. Urine samples will be collected from participants for cotinine testing to evaluate validity of self-reported smoking behavior in a sample of individuals participating in a lung cancer screening trial.
Specific Aim #3: The tertiary aim of this research is to examine concordance between self-report smoking behavior and a biochemical index of smoking behavior in a sub-sample of study participants. Urine samples will be collected from participants for cotinine testing to evaluate validity of self-reported smoking behavior in a sample of individuals participating in a lung cancer screening trial.
Hypothesis #3: Self-report smoking behavior will be concordant with the biochemical index of smoking.
Specific Aim #4: The fourth aim is to establish an empirical foundation for designing lung cancer screening programs as well as developing interventions which: (a) promote participation and retention; and (b) minimize negative behavioral, cognitive, and affective responses to lung cancer screening.
Publications:
1. Validity of self-reported smoking status among participants in a lung cancer screening trial
2. Participant Adherence in a RCT of Lung Cancer Screening: Results from Baseline to Year 1 (in press)
3.
4.
 Facebook
Facebook Twitter
Twitter Linkedin
Linkedin