Transcranial Magnetic Stimulation
NeuroStar TMS Therapy is a new treatment cleared by the US Food and Drug Administration (FDA) for patients suffering from depression* who have not achieved satisfactory improvement from prior antidepressant treatment. TMS stands for “transcranial magnetic stimulation.”
NeuroStar TMS Therapy device
TMS Therapy is a treatment that can be performed in a psychiatrist’s office, under their supervision, using a medical device called the NeuroStar TMS Therapy system. NeuroStar TMS Therapy is:
Non-invasive, meaning that it does not involve surgery. It does not require any anesthesia or sedation, as the patient remains awake and alert during the treatment.
Non-systemic, meaning that it is not taken by mouth and does not circulate in the blood stream throughout the body.
The typical initial treatment course consists of 5 treatments per week over a 4-6 week period, for an average of 20-30 total treatments. Each treatment session lasts approximately 40 minutes.
Through a treatment coil, the NeuroStar TMS Therapy system generates highly concentrated, magnetic fields which turn on and off very rapidly. These magnetic fields are the same type and strength as those produced by a magnetic resonance imaging (MRI) machine.
The treatment coil is applied to the head above the left prefrontal cortex. This part of the brain is involved with mood regulation, and therefore is the location where the magnetic fields are focused. These magnetic fields do not directly affect the whole brain; they only reach about 2-3 centimeters into the brain directly beneath the treatment coil. As these magnetic fields move into the brain, they produce very small electrical currents. These electrical currents activate cells within the brain which are thought to release neurotransmitters.
NeuroStar TMS Therapy has been demonstrated to be safe and tolerable.
Over 10,000 active treatments were safely performed with NeuroStar TMS Therapy during the clinical trials.
- No side effects such as weight gain, sexual problems, stomach problems, sleepiness, or dry mouth were seen during trials
- There were no negative effects on memory or ability to concentrate
The most commonly reported side effect related to treatment was scalp pain or discomfort during the treatment session. This side effect was generally mild to moderate, and occurred less frequently after the first week of treatment. Less than 5% of patients treated with NeuroStar TMS Therapy discontinued treatment due to side effects.
NeuroStar TMS Therapy has been demonstrated to be effective in Major Depressive Disorder.*
Efficacy was established in a controlled clinical trial comparing active treatment with the NeuroStar TMS Therapy system to an inactive device. Patients treated with active NeuroStar TMS Therapy experienced an average reduction in their depression symptom score of 22.1% compared to a 9% average reduction in patients receiving inactive treatment. NeuroStar-treated patients also experienced significant improvement in anxiety, appetite changes, aches and pains, and lack of energy associated with depression.
In an open label trial, which is most like a real world clinical practice, approximately 1 out of 2 patients treated with NeuroStar TMS Therapy experienced significant improvement in depression symptoms. Approximately 1 out of 3 patients treated with NeuroStar TMS Therapy experienced complete symptom relief at the end of 6 weeks. As with any antidepressant treatment, patients should be monitored for symptoms of worsening depression.
NeuroStar TMS Therapy is an appropriate treatment option for adult patients with Major Depressive Disorder who have failed to achieve satisfactory improvement from one prior antidepressant medication at or above the minimal effective dose and duration in the current episode.
In clinical trials, half of the patients had been treated with at least four medication treatment attempts, one of which was at an adequate dose and duration.
NeuroStar TMS Therapy has not been studied in patients who have not received prior antidepressant treatment. Efficacy has not been established in patients who have failed to receive benefit from two or more prior antidepressant medications at minimal effective dose and duration in the current episode.
Not all patients are appropriate candidates for NeuroStar TMS Therapy. To determine if NeuroStar TMS Therapy may be right for you, talk with your physician.
NeuroStar TMS Therapy should not be used in patients with implanted metallic devices or non-removable metallic objects in or around the head (for example, metal plates in the skull, aneurysm coils, etc.) because serious injury could result.
Patients with braces and metal fillings are acceptable for treatment; however, patients with other metal within their mouth should discuss this with their physician.
NeuroStar TMS Therapy should not be used in patients with implants controlled by physiological signals. This includes pacemakers, implantable cardioverter defibrillators (ICDs) and vagus nerve stimulators (VNS).
Use the links below to learn more about this treatment option.
- VIDEOS:
Medical Watch: Depression Treatment video - WGNtv.com - Using magnets to fight depression video - ABC news
- WEBSITES:
TMS is one of the Top 10 health innovations of 2009 - Magnetic Relief For Depression? - The Washington Post
- NeuroStar Depression Therapy Cleared - Targets nerve cells with magnetic pulses - U.S. News & World Report
- A New Approach to Treating Intractable Cases of Depression - Wall Street Journal
- Beyond antidepressants: There are other treatments - Los Angeles Times
- 14 Medical Pioneers Who Aren't Holding Back - U.S. News & World Report
*NeuroStar TMS Therapy® is indicated for the treatment of Major Depressive Disorder in adult patients who have failed to achieve satisfactory improvement from one prior antidepressant medication at or above the minimal effective dose and duration in the current episode.

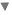 How TMS works
How TMS works