Proposal Documents
The IACUC regulates the use of animals by requiring investigators to submit a "Proposal to Use Laboratory Animals in Research and Teaching" and controls the use of animal tissue through the form entitled "Request to Use Fresh or Frozen Animal Tissues in Research and Teaching." The IACUC proposal is periodically revised to enhance Committee review, improve project design, and provide information that will heighten the awareness of UofL scientists regarding humane animal care and use.
Review Process
The IACUC reviews all animal use proposals and requests to use animal tissue, including those involved in pilot and/or internally funded research. Proposals must be submitted by Project Directors prior to the start of related research or teaching activities. Each USDA-regulated animal proposal is subject to an annual review; continued approval may be granted during the annual review process. A newly revised application must be submitted at the end of three years.
Proposals are initially submitted to IACUC via the web-based 
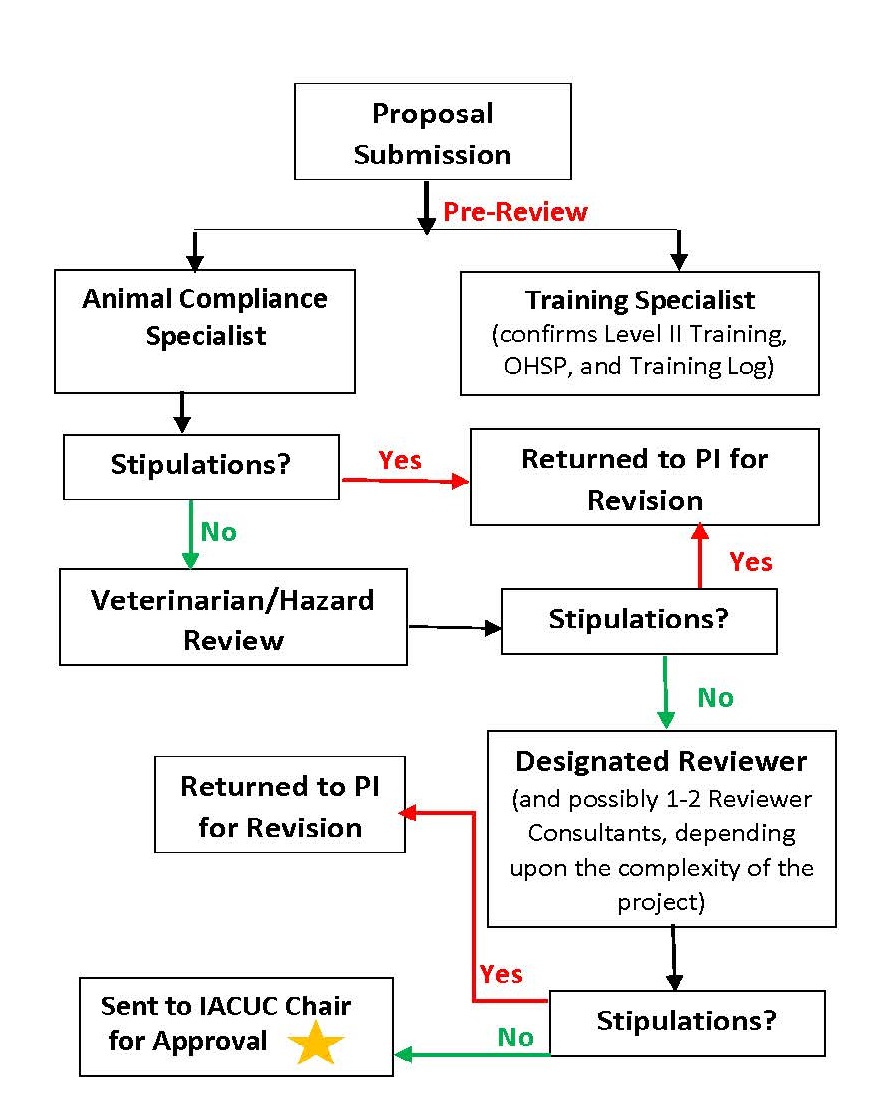
The Office of Research Services prepares weekly action reports that are forwarded to all IACUC members. These reports contain a summary list of all proposals that are under review (to include project director, title, species, date submitted, status, DR, and lay summary). Any IACUC member requesting additional information is provided a copy of the Proposal in question. At any time during the review process, an IACUC member may request Full Committee Review (FCR). Prior to any official decision, FCR requires a discussion of the Proposal during a monthly, convened IACUC meeting.
While encompassing all aspects of the Proposal, Committee review places special emphasis on the significance of study goals weighed against the potential harm that may be imposed on animals that serve as models for human disease. Decisions involving Proposal disposition (approval, contingent approval, or disapproval) are made only after consideration has been given to other research methods that may not involve animals and a written assurance that proposed activities do not unnecessarily duplicate previous experiments. The Committee also addresses the ethics of animal or tissue use by considering the animal and human health benefits and/or other societal good that is likely to accrue from the proposed research. With respect to the review of protocols that have a potential to cause pain or distress, committee members focus on the proposal section devoted to the consideration of alternatives to painful procedures. A written narrative description of the methods and sources used to determine that alternatives were not available is required before a project may receive IACUC approval. When the Committee is satisfied that the project director has addressed the issue of alternatives and determined that there is no way to avoid the potential for pain and distress, the use of analgesics and anesthetics is reviewed. All proposals require the use of appropriate methods and/or agents to minimize pain or distress and a detailed description of sedatives, analgesics and/or anesthetics to be used must be given.
Note: The UofL IACUC will not consider proposals that are characterized by the likelihood of severe, prolonged, or unrelieved pain or distress without comprehensive and explicit scientific justification. Such protocols would also be placed on higher priority for thorough scrutiny during the annual review and semi-annual laboratory inspection processes.
