UofL scientists identify a critical pathway to improve muscle repair
Researchers at the University of Louisville have discovered a mechanism involved in skeletal muscle repair that may enable clinicians to boost the effectiveness of adult stem cell therapies for diseases such as muscular dystrophy. The research, published today in the Journal of Clinical Investigation, describes the role of TNF receptor-associated factor 6 (TRAF6), an adaptor protein and E3 ubiquitin ligase, in ensuring the vitality of stem cells that regenerate muscle tissue.
Specialized stem cells known as satellite cells reside in skeletal muscle in an inactive state. When muscle injury occurs, a complex chain of signals prompts the satellite cells to awaken and generate new muscle cells to repair the injury. Previous research had shown that Pax7 (a paired-box transcription factor) is essential to this regeneration. When Pax7 is missing or reduced, the satellite cells undergo premature differentiation, or lose their stem properties and their ability to regenerate injured muscles.
In their research, authors Sajedah M. Hindi, Ph.D., and Ashok Kumar, Ph.D., discovered that removing TRAF6 depletes Pax7, resulting in reduced muscle regeneration in both normal and Duchenne muscular dystrophy (DMD) mouse models. Hindi, a post-doctoral fellow, and Kumar, professor and distinguished university scholar in UofL’s Department of Anatomical Sciences and Neurobiology, believe this is because TRAF6 is upstream from Pax7 in the signaling process involved in muscle repair and orchestrates multiple signals controlling the muscle regeneration process.
“We have discovered a pathway by which the Pax7 and myogenic potential of satellite cells is regulated. The protein TRAF6 is a very important adaptor protein that is involved in multiple signaling pathways and its functions are important to maintain the stemness of satellite cells in adults,” Kumar said.
“In normal conditions, skeletal muscle is a self-healing tissue and can recover promptly from most trauma because of the satellite cells. But in disease conditions like muscular dystrophies, satellite cells can’t keep up with repeated cycles of injury and are ultimately exhausted or functionally impaired,” Hindi said. “Our next step is to see if this functional impairment is partially due to lack of TRAF6 signaling in satellite cells. If so, we are thinking we can take a patient’s stem cells, restore the TRAF6 activity, put them back and boost their regenerative potential.”
Kumar and Hindi believe their research ultimately will lead to improved treatments for muscle wasting diseases such as muscular dystrophy, ALS, cancer cachexia, diabetes, heart disease and others.
“Right now the problem in donor stem cell therapy is that we inject the stem cells into the patient but most of the stem cells don’t proliferate very well, so they repair very little part of the muscle,” Kumar said. “But if you have stem cells that are over expressing this protein TRAF6, they may proliferate longer and they may repair the muscle much more effectively.”
Research reported in this press release was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Aging of the National Institutes of Health under award numbers R01AR059810, R01AR068313, R01AG029623 and F31AG046950.
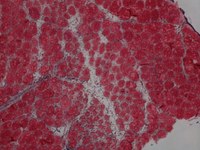
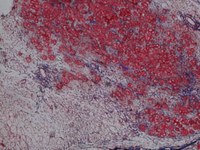
IMAGES: TRAF6 fl/fl (top) are control injured muscle whereas TRAF6scko (bottom) are from satellite cell-specific TRAF6- knockout mice which show drastic deficit in muscle regeneration due to lack of TRAF6.
November 30, 2015
 Facebook
Facebook Twitter
Twitter Linkedin
Linkedin