The Envirome and Health
Contents
Introduction
Our Health and well-being depends upon our envirome.
Like all other living things, we are entrained to the cycles of night and day, and dyssynchrony with this primordial cycle increases the risk of heart disease and cancer. Like plants, we need sunlight for photosynthesis of vitamin D, and our physiology and physiological adaptations vary with altitude and latitudes. Plants, animals, and microbes in our environment are sources of food, but also disease and infection.
Throughout history, we have sought to minimize the impact of the natural environment by building villages, houses, and cities. We have also developed agriculture, animal husbandry, and technology. We have created complex societies with varying cultures, occupations, hierarchies, and power structures. Feature of these social institutions profoundly affect our physical and mental health. Pollution, discrimination, restricted access to health care, and health inequities that arise from our social organization significantly affect how well and how long we live.
From the society they live in, individuals create their own personal environments. These microenvironments are formed by chance, circumstance, and choice. They are characterized by the conditions closest to us, such as our house, our family, and our income as well as our choices that relate not only to nutrition and physical activity, but also to the use of tobacco, alcohol, and recreational drugs. Although personal environments exist within the larger, shared, and more public social environments, they differ among individuals living in the same society and culture.
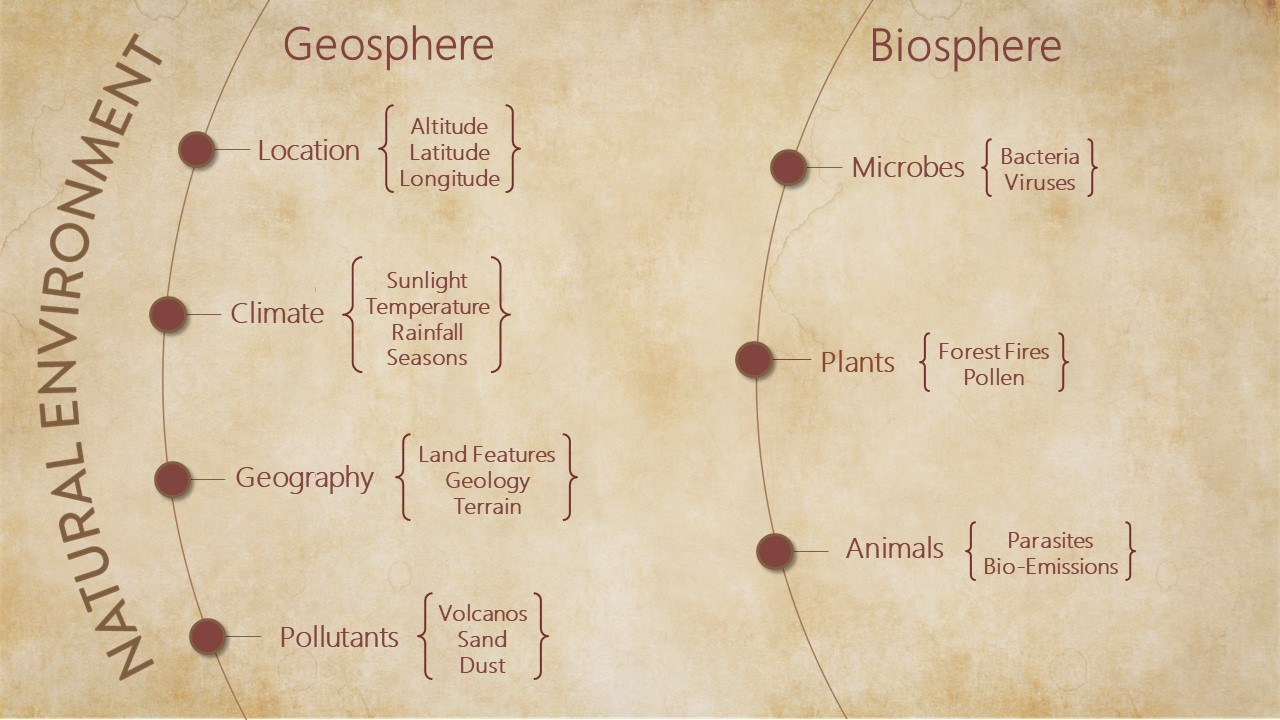
The Natural Environment
The natural environment is all that is non-human in our envirome. It includes microbes, plants and animals, the cycles of night and day, and the rhythms of seasons, as well as altitude, latitude, rainfall, and sunshine – aspects of nature that constantly and profoundly affect our lives.
Circadian Rhythms
The day/night cycle is a fundamental, invariant feature of the natural environment. All life is entrained to this cycle, which in turn exerts a pervasive control over both plants and animals. In mammals, sunlight regulates the master clock in the suprachiasmatic nucleus, which synchronizes the light-insensitive peripheral clocks to coordinate a 24 h cycle. This day/ night cycle controls both cardiovascular health and function; heart rate and blood pressure are lowest at night and during sleep and begin to rise before waking-up, coinciding with a period of vagal dominance, in anticipation of daytime activities. Circadian cues also regulate the expression of cardiovascular genes and the abundance of cardiovascular proteins,1, 2 as well as the levels of neurohormones that regulate cardiovascular function, such angiotensin II, renin, aldosterone, growth hormone and atrial naturetic peptide.3
- Martino T, Arab S, Straume M, Belsham DD, Tata N, Cai F, Liu P, Trivieri M, Ralph M, Sole MJ. Day/night rhythms in gene expression of the normal murine heart. J Mol Med. 2004; 82:256–64. [PubMed: 14985853]
- McNamara P, Seo SB, Rudic RD, Sehgal A, Chakravarti D, FitzGerald GA. Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: a humoral mechanism to reset a peripheral clock. Cell. 2001; 105:877–89. [PubMed: 11439184]
- Martino TA, Sole MJ. Molecular time: an often overlooked dimension to cardiovascular disease. Circ Res. 2009; 105:1047–61. [PubMed: 19926881]
Seasons
In most places, the natural environment is characterized not only by diurnal rhythms, but by a change in seasons as well. In most locations, this leads to wide variations in temperature and humidity as well as the length of day. A change of season alters sunlight exposure, physical activity, and feeding behavior; changes which, by modifying physiological responses and metabolism, could affect cardiovascular function and disease. In both Northern and Southern hemispheres, the levels of blood pressure and plasma HDL, LDL, and glucose are slightly higher in winter than in summer,1 and it has been reported that more patients on statin therapy achieve their target LDL level in summer than in winter, suggesting that plasma lipoprotein metabolism in humans may be regulated by the seasons.2 Similar seasonal variations have been reported in animals. In European badgers, for instance, the plasma cholesterol levels are 650% higher in winter than in summer; LDL levels peak in autumn/winter, while HDL predominates in early spring.3 In humans, seasonal variations have also been observed in fibrinogen,4 tissue plasminogen activator antigen, and von Willebrand factor.5
- Marti-Soler H, Gubelmann C, Aeschbacher S, Alves L, Bobak M, Bongard V, Clays E, de Gaetano G, Di Castelnuovo A, Elosua R, Ferrieres J, Guessous I, Igland J, Jorgensen T, Nikitin Y, O'Doherty MG, Palmieri L, Ramos R, Simons J, Sulo G, Vanuzzo D, Vila J, Barros H, Borglykke A, Conen D, De Bacquer D, Donfrancesco C, Gaspoz JM, Giampaoli S, Giles GG, Iacoviello L, Kee F, Kubinova R, Malyutina S, Marrugat J, Prescott E, Ruidavets JB, Scragg R, Simons LA, Tamosiunas A, Tell GS, Vollenweider P, Marques-Vidal P. Seasonality of cardiovascular risk factors: an analysis including over 230 000 participants in 15 countries. Heart. 2014; 100:1517–23. [PubMed: 24879630]
- Tung P, Wiviott SD, Cannon CP, Murphy SA, McCabe CH, Gibson CM. Seasonal variation in lipids in patients following acute coronary syndrome on fixed doses of Pravastatin (40 mg) or Atorvastatin (80 mg) (from the Pravastatin or Atorvastatin Evaluation and Infection TherapyThrombolysis In Myocardial Infarction 22 [PROVE IT-TIMI 22] Study). Am J Cardiol. 2009; 103:1056–60. [PubMed: 19361589]
- Laplaud PM, Beaubatie L, Maurel D. A spontaneously seasonal hypercholesterolemic animal: plasma lipids and lipoproteins in the European badger (Meles meles L.). J Lipid Res. 1980; 21:724–38. [PubMed: 7419984]
- Hermida RC, Calvo C, Ayala DE, Lopez JE, Fernandez JR, Mojon A, Dominguez MJ, Covelo M. Seasonal variation of fibrinogen in dipper and nondipper hypertensive patients. Circulation. 2003; 108:1101–6. [PubMed: 12912809]
- Rudnicka AR, Rumley A, Lowe GD, Strachan DP. Diurnal, seasonal, and blood-processing patterns in levels of circulating fibrinogen, fibrin D-dimer, C-reactive protein, tissue plasminogen activator, and von Willebrand factor in a 45-year-old population. Circulation. 2007; 115:996–1003. [PubMed: 17296859]
Sunlight
Seasonal and latitudinal variations in vitamin D levels have been associated with geographic and seasonal variations in blood pressure. With increasing distance from the equator, there is a progressive increase in blood pressure, which correlates with a gradual fall in ambient UVB radiation.1 The prevalence of hypertension shows a similar latitudinal distribution. Moreover, blood pressure is higher in winter,1, 2 when UVB levels are low and decreases in summer when sunnier days arrive. Although a causal relationship between blood pressure and sunlight remains to be fully clarified (see below), it has been reported that exposure to UVB radiation3 skin tanning in salons4 or treatment with high-dose vitamin D25 reduces blood pressure. In addition to blood pressure, vitamin D regulates other cardiovascular functions as well. All cardiovascular tissues express vitamin D receptor (VDR),6 which regulates the expression of nearly 200 genes.6 Overall, 3% of the human genome is regulated by vitamin D.
- Rostand SG. Ultraviolet light may contribute to geographic and racial blood pressure differences. Hypertension. 1997; 30:150–6. [PubMed: 9260973]
- Argiles A, Mourad G, Mion C. Seasonal changes in blood pressure in patients with end-stage renal disease treated with hemodialysis. N Engl J Med. 1998; 339:1364–70. [PubMed: 9801397]
- Kokot F, Schmidt-Gayk H, Wiecek A, Mleczko Z, Bracel B. Influence of ultraviolet irradiation on plasma vitamin D and calcitonin levels in humans. Kidney Int Suppl. 1989; 27:S143–6. [PubMed: 2636650]
- Krause R, Buhring M, Hopfenmuller W, Holick MF, Sharma AM. Ultraviolet B and blood pressure. Lancet. 1998; 352:709–10.
- Sugden A, Smith J, Pennisi E. The future of forests. Science. 2008; 320:1435.
- Lee JH, O'Keefe JH, Bell D, Hensrud DD, Holick MF. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? J Am Coll Cardiol. 2008; 52:1949–56. [PubMed: 19055985]
Altitude
Altitude is additional aspect of the natural environment that has an important bearing upon human health. Nearly 400 million people live in areas more than 1500 m (4,900 feet) above sea level1 and human populations living in these areas have developed significant anatomical, physiologic, and metabolic adaptation to cold temperature and low oxygen levels. Of the several highland populations, native Tibetans and Nepalese Sherpas are best adapted to high altitudes. Tibetans have the oldest altitude ancestry, and through successive generations they have attained a high grade of adaptation to high altitudes. They rarely exhibit systolic hypertension and have lower levels of cholesterol and apoB than sea level dwellers.2 They also show lower pulmonary pressure in response to exercise with less increase in ventilation rates and better preservation of cardiac output. In contrast, Andean natives who have a shorter history of living at high altitudes are less well adapted and they show greater muscularlization of the distal pulmonary arterial branches and LV hypertrophy. 3
- Burtscher M. Effects of living at higher altitudes on mortality: a narrative review. Aging Dis. 2014; 5:274–80. [PubMed: 25110611]
- Fujimoto N, Matsubayashi K, Miyahara T, Murai A, Matsuda M, Shio H, Suzuki H, Kameyama M, Saito A, Shuping L. The risk factors for ischemic heart disease in Tibetan highlanders. Jpn Heart J. 1989; 30:27–34. [PubMed: 2724529]
- Arias-Stella J, Saldana M. The Terminal Portion of the Pulmonary Arterial Tree in People Native to High Altitudes. Circulation. 1963; 28:915–25. [PubMed: 14079195]
Greenspaces
Throughout evolution, interaction with natural vegetation has been an invariant feature of the human environment. Even though the project of civilization is to immure human activity in artificial, built environments, humans display innate biophilic preferences. Believed to be a product of evolution, these tendencies counter the instinctive fear of natural threats and predators, and may underlie the well-known restorative effects of nature and natural vegetation on mental health. Whether or not interactions with nature are also important for physical health remains less clear, but an association between vegetation and physical health is consistent with the results of many recent studies, showing that even in modern urban environments of sprawling metropolises and congested conurbations, residential proximity to vegetation is associated with lower levels of stress, diabetes, stroke and CVD.1, 2 Living in artificial environments minimizes contact with natural elements such as sunlight, animals and plants that have salutary effects on health.3 Previous studies have shown that residential proximity to vegetation is associated with lower levels of stress, diabetes, stroke and CVD,1, 2 and individual level data indicate that children living in greener areas have lower levels of asthma,4 blood pressure,5 and insulin resistance.6 In adults, residential proximity to greenness has been associated with better general health, enhanced social support, and physical activity.1, 7
- Dadvand P, Bartoll X, Basagana X, Dalmau-Bueno A, Martinez D, Ambros A, Cirach M, TrigueroMas M, Gascon M, Borrell C, Nieuwenhuijsen MJ. Green spaces and General Health: Roles of mental health status, social support, and physical activity. Environ Int. 2016; 91:161–7. [PubMed: 26949869]
- James P, Banay RF, Hart JE, Laden F. A Review of the Health Benefits of Greenness. Curr Epidemiol Rep. 2015; 2:131–142. [PubMed: 26185745]
- Bhatnagar, A. Environmental Cardiology: Pollution and Heart Disease. Royal Society of Chemistry; 2011.
- Lovasi GS, Quinn JW, Neckerman KM, Perzanowski MS, Rundle A. Children living in areas with more street trees have lower prevalence of asthma. J Epidemiol Community Health. 2008; 62:647– 9. [PubMed: 18450765]
- Markevych I, Thiering E, Fuertes E, Sugiri D, Berdel D, Koletzko S, von Berg A, Bauer CP, Heinrich J. A cross-sectional analysis of the effects of residential greenness on blood pressure in 10-year old children: results from the GINIplus and LISAplus studies. BMC Public Health. 2014; 14:477. [PubMed: 24886243]
- Thiering E, Markevych I, Bruske I, Fuertes E, Kratzsch J, Sugiri D, Hoffmann B, von Berg A, Bauer CP, Koletzko S, Berdel D, Heinrich J. Associations of Residential Long-Term Air Pollution Exposures and Satellite-Derived Greenness with Insulin Resistance in German Adolescents. Environ Health Perspect. 2016; 124:1291–8. [PubMed: 26863688]
- Maas J, Verheij RA, Groenewegen PP, de Vries S, Spreeuwenberg P. Green space, urbanity, and health: how strong is the relation? J Epidemiol Community Health. 2006; 60:587–92. [PubMed: 16790830]
Social Environment
Our social interactions, our history, and our culture, make up our social environment. The social environment deeply transforms the influence of the natural environment. By interacting with nature, we develop agriculture, invent technology, and fashion food networks. Our interactions with each other create civic and social organizations housed in built structures such as cities, roads, and parks. By generating noise, pollutants, and toxins, these environments threaten human health, and by their varied structures, they influence healthcare access, social cohesion, health disparities, and socioeconomic status.
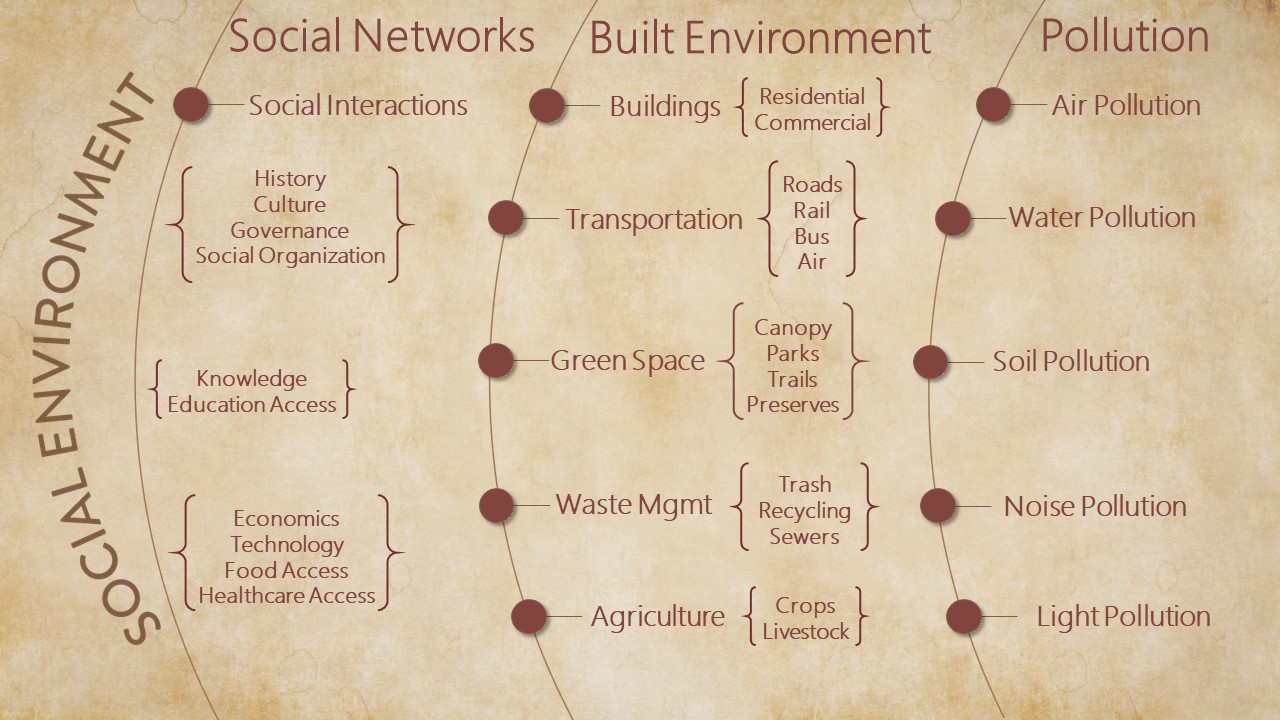
The Built Environment
Cohesive communities fashion rich social environments consisting of houses, cities, and roads, with the purpose of promoting human health and optimizing human flourishing. They create artificial environments to protect against the elements; the vagaries of nature; and the threat of natural predators and pests. The creation of these artificial, but safe, environments may have been particularly critical for human evolution, as humans have long, protracted childhoods, when they are much more vulnerable to natural threats than other animals. However, built environments alienate humans from nature and they modify the influence of the natural environment on human health and development. Artificial environments create new problems, such as crowding, noise, and pollution; problems that ultimately limit health and promote disease.
By moderating or modifying the influence of local ecology and by creating artificial, non-natural living spaces, the built environment could either promote or prevent disease. It could prevent disease by creating sanitary, climate regulated, safe spaces, but it could also promote disease by generating unconducive living conditions.
The clearest impact of the built environment could be seen with obesity. Meta-analyses of over 60 studies show that aspects of the built environment are positively correlated with obesity1, particularly in disadvantaged groups2. Strongest evidential support was found for food stores (supermarkets instead of smaller grocery stores), places to exercise, and safety. Each of these neighborhood characteristics were found to be correlated with body mass index2. Greater neighborhood physical activity resources were associated with lower insulin resistance3 and high walkability neighborhoods were associated with decreases in weight and waist circumference4. These measures of obesity were also associated with high density of fast-food restaurants4. For neighborhoods with a high-density of fast-food restaurants an odds ratio of 1.8 has been reported5. Each quartile increase in land-use mix has been found to be associated with a 12% reduction in the likelihood of obesity.6 Moreover, each additional hour spent in car per day was associated with a 6% increase in obesity risk and each kilometer walked per day with a 55% reduction in the likelihood of obesity.
- Papas MA, Alberg AJ, Ewing R, Helzlsouer KJ, Gary TL, Klassen AC. The built environment and obesity. Epidemiol Rev. 2007; 29:129–43. [PubMed: 17533172]
- Lovasi GS, Hutson MA, Guerra M, Neckerman KM. Built environments and obesity in disadvantaged populations. Epidemiol Rev. 2009; 31:7–20. [PubMed: 19589839]
- Auchincloss AH, Diez Roux AV, Brown DG, Erdmann CA, Bertoni AG. Neighborhood resources for physical activity and healthy foods and their association with insulin resistance. Epidemiology. 2008; 19:146–57. [PubMed: 18091002]
- Li F, Harmer P, Cardinal BJ, Bosworth M, Johnson-Shelton D, Moore JM, Acock A, Vongjaturapat N. Built environment and 1-year change in weight and waist circumference in middle-aged and older adults: Portland Neighborhood Environment and Health Study. Am J Epidemiol. 2009; 169:401–8. [PubMed: 19153214]
- Li F, Harmer P, Cardinal BJ, Bosworth M, Johnson-Shelton D. Obesity and the built environment: does the density of neighborhood fast-food outlets matter? Am J Health Promot. 2009; 23:203–9. [PubMed: 19149426]
- Frank LD, Andresen MA, Schmid TL. Obesity relationships with community design, physical activity, and time spent in cars. Am J Prev Med. 2004; 27:87–96. [PubMed: 15261894]
Pollution
The modern environment is awash with synthetic chemicals and pollutants. By some estimates, > 30,000 synthetic chemicals are in current use, of these at least 5,500 are produced at > 100 tons per year.1 Almost all major rivers and lakes show significant contamination by synthetic chemicals, pesticides or metals. Pesticides such as lindane, chlordane and DTT from Asia have been detected in Canadian Rockies and mercury generated by human activity has been detected in Arctic wild-life.2 As a result, there are no pristine, un-polluted places left on the entire planet. High levels of pollutants are also released in the air. Although the level of air pollution in the developed world today is much lower than during its peak in the 1950s–1970s, the levels of air pollution in the developing world remain extraordinarily high.
Most air pollution is a mixture of complex aerosols containing both particles and gases. Particulate air pollution consists of particulate matter (PM), which when analyzed for mass fall into two peaks, corresponding to coarse particles (10-2.5 µm) and fine particles (0.1 to 2.5 µm). The fine particle mode also contains a small fraction of ultrafine particles, which despite its modest contribution to the overall volume of PM, contains the largest number of particles. Aerosols emitted in the environment directly are composed mostly of minerals, soot, salt particles, pollens and spores, whereas secondary aerosols are generated by sulfates, nitrates and organic compounds. In addition, both indoor and outdoor air contains a variety of gaseous pollutants, such as volatile organic chemicals (VOCs), nitrogen and sulfur oxides, and ozone. The composition of ambient air particles and gases in the atmosphere varies with meteorological conditions, local sources, geography, and seasons and could be quite complex, making it difficult to link constituents with health effects.
The WHO estimates that globally air pollution could be linked to 7 million premature deaths per year. This includes 1.6 million deaths in China and 1.3 million deaths in India. Estimates of premature mortality in the US from outdoor air pollution vary from 55,0003 to 200,000.4 In its health impact, air pollution rivals the effects of hypertension, smoking and physical inactivity.5 Exposure to air pollution is pervasive and in some places, ubiquitous and unavoidable. In parts of the developing world, >95% of the urban population lives in cities where the levels of air pollution exceeds the air quality guidelines of the WHO.5 Notably, most of this mortality in developing countries, such as China and India, is associated with residential and commercial energy use, which has been linked to over 10 million excessive deaths.3 This estimate, however, does not include an additional 3.54 million deaths per year due to household air pollution due to biomass burning. In the US and Western Europe, agriculture, power generation and land traffic appear to be the major source categories. Agriculture, which contributes to PM2.5 formation by releasing ammonia from fertilizer use and domestic animals, contributes to nearly 20% of the global burden of outdoor air pollution, corresponding to an estimated 6.6 million deaths per year worldwide.3
While exposure to PM2.5 has been linked premature mortality due to respiratory diseases and cancer, between 70–80% of premature deaths due to exposure to PM2.5 are due to cardiovascular causes.6 Reasons for the unique vulnerability of cardiovascular tissues to air pollution remain unclear, but extensive evidence has documented acute exacerbations of cardiovascular events upon exposure to particulate air pollution and chronic increase in CVD in individuals exposed recurrently to air pollution. Even brief exposures to polluted air are associated with MI, stoke, arrhythmias, atrial fibrillation and hospitalization for exacerbation of heart failure in susceptible individuals.3-5 There is equally strong evidence suggesting that chronic and persistent exposure to air pollution increases the progression of atherosclerotic lesions and has adverse effects on blood pressure regulation, peripheral thrombosis, endothelial function, and insulin sensitivity.5-8
In addition to individual susceptibility factors, vulnerability to ambient air pollution is also moderated by environmental factors, such as the built environment, noise, ambient temperature, neighborhood greenspaces, and proximity to major roadways or co-exposure to other pollutants and toxins. Individuals who live within close proximity to major roadways have been found to have increased carotid-intima thickness with increased exposure to traffic9 and increased CVD risk as reflected by an increase in chronic inflammation,10 circulating levels of angiogenic cells,11 abdominal adiposity,12 and incident hypertension.13 Roadway proximity has been associated with elevated risk of acute myocardial infarction,14 sudden cardiac death,15 fatal coronary disease,15 post-stroke mortality,16 and mortality risk after hospitalization with acute heart failure.17
- Hartung T. Toxicology for the twenty-first century. Nature. 2009; 460:208–12. [PubMed: 19587762]
- Wilkening KE, Barrie LA, Engle M. Atmospheric science. trans-Pacific air pollution. Science. 2000; 290:65–7. [PubMed: 11183151]
- Lelieveld J, Evans JS, Fnais M, Giannadaki D, Pozzer A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature. 2015; 525:367–71. [PubMed: 26381985]
- Caiazzo F, Ashok A, Waitz IA, Yim SH, Barrett SR. Air pollution and early deaths in the United States. Part I: Quantifying the impact of major sectors in 2005. Atmospheric Environment. 2013; 79:198–208.
- Cosselman KE, Navas-Acien A, Kaufman JD. Environmental factors in cardiovascular disease. Nat Rev Cardiol. 2015; 12:627–42. [PubMed: 26461967]
- Bhatnagar A. Environmental cardiology: studying mechanistic links between pollution and heart disease. Circ Res. 2006; 99:692–705. [PubMed: 17008598]
- Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC Jr, Whitsel L, Kaufman JD. American Heart Association Council on E, Prevention CotKiCD, Council on Nutrition PA and Metabolism. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010; 121:2331–78. [PubMed: 20458016]
- Gold DR, Mittleman MA. New insights into pollution and the cardiovascular system: 2010 to 2012. Circulation. 2013; 127:1903–13. [PubMed: 23648681]
- Bauer M, Moebus S, Mohlenkamp S, Dragano N, Nonnemacher M, Fuchsluger M, Kessler C, Jakobs H, Memmesheimer M, Erbel R, Jockel KH, Hoffmann B. Group HNRSI. Urban particulate matter air pollution is associated with subclinical atherosclerosis: results from the HNR (Heinz Nixdorf Recall) study. J Am Coll Cardiol. 2010; 56:1803–8. [PubMed: 21087707]
- Hoffmann B, Moebus S, Dragano N, Stang A, Mohlenkamp S, Schmermund A, Memmesheimer M, Brocker-Preuss M, Mann K, Erbel R, Jockel KH. Chronic residential exposure to particulate matter air pollution and systemic inflammatory markers. Environ Health Perspect. 2009; 117:1302–8. [PubMed: 19672412]
- DeJarnett N, Yeager R, Conklin DJ, Lee J, O'Toole TE, McCracken J, Abplanalp W, Srivastava S, Riggs DW, Hamzeh I, Wagner S, Chugh A, DeFilippis A, Ciszewski T, Wyatt B, Becher C, Higdon D, Ramos KS, Tollerud DJ, Myers JA, Rai SN, Shah J, Zafar N, Krishnasamy SS, Prabhu SD, Bhatnagar A. Residential Proximity to Major Roadways Is Associated With Increased Levels of AC133+ Circulating Angiogenic Cells. Arterioscler Thromb Vasc Biol. 2015; 35:2468–77. [PubMed: 26293462]
- Li W, Dorans KS, Wilker EH, Rice MB, Schwartz J, Coull BA, Koutrakis P, Gold DR, Fox CS, Mittleman MA. Residential proximity to major roadways, fine particulate matter, and adiposity: The framingham heart study. Obesity (Silver Spring). 2016; 24:2593–2599. [PubMed: 27804220]
- Kingsley SL, Eliot MN, Whitsel EA, Wang Y, Coull BA, Hou L, Margolis HG, Margolis KL, Mu L, Wu WC, Johnson KC, Allison MA, Manson JE, Eaton CB, Wellenius GA. Residential proximity to major roadways and incident hypertension in post-menopausal women. Environ Res. 2015; 142:522–8. [PubMed: 26282224]
- Tonne C, Melly S, Mittleman M, Coull B, Goldberg R, Schwartz J. A case-control analysis of exposure to traffic and acute myocardial infarction. Environ Health Perspect. 2007; 115:53–7. [PubMed: 17366819]
- Hart JE, Chiuve SE, Laden F, Albert CM. Roadway proximity and risk of sudden cardiac death in women. Circulation. 2014; 130:1474–82. [PubMed: 25332277]
- Wilker EH, Mostofsky E, Lue SH, Gold D, Schwartz J, Wellenius GA, Mittleman MA. Residential proximity to high-traffic roadways and poststroke mortality. J Stroke Cerebrovasc Dis. 2013; 22:e366–72. [PubMed: 23721619]
- Medina-Ramon M, Goldberg R, Melly S, Mittleman MA, Schwartz J. Residential exposure to traffic-related air pollution and survival after heart failure. Environ Health Perspect. 2008; 116:481–5. [PubMed: 18414630]
Environmental Noise
Like air pollution, noise is another important environmental factor that has an important bearing on cardiovascular health and disease. Noise generated from several sources, such as roadway traffic, railroads, and aircrafts interferes with communication, causes annoyance and disturbs sleep. In the US, nearly 46% of the population (145.5 million) is exposed to noise at levels exceeding 55 dBA LDN (weighted day-night 24h average noise level) and 43.8 million individuals are exposed to noise levels exceeding 65 dBA LDN.1 In Europe, 40% of the population is exposed to road traffic noise exceeding 55 dBA LDN and >30% to 55 dB at night.2 Constant exposure to noise induces stress, and affects cognitive function, autonomic homeostasis and sleep quality, all of which could increase CVD risk. Direct exposure studies with humans have shown that simulated traffic noise increases blood pressure, heart rate and cardiac output; effects that are likely to be mediated by the release of catecholamines, cortisol, and other stress hormones. Similarly, exposure to air craft noise, particularly at night, induces endothelial dysfunction measured by flow-dependent dilation, as well as an increase in blood pressure.2 In animal models, chronic exposure to continuous noise (80–100 dB) has been reported to increase heart rate and mean systemic arterial blood pressure, functional changes that were associated with an increase in plasma corticosterone, adrenaline and endothelin-1.3
- Swinburn TK, Hammer MS, Neitzel RL. Valuing Quiet: An Economic Assessment of U.S. Environmental Noise as a Cardiovascular Health Hazard. Am J Prev Med. 2015; 49:345–53. [PubMed: 26024562]
- Munzel T, Gori T, Babisch W, Basner M. Cardiovascular effects of environmental noise exposure. Eur Heart J. 2014; 35:829–36. [PubMed: 24616334]
- Said MA, El-Gohary OA. Effect of noise stress on cardiovascular system in adult male albino rat: implication of stress hormones, endothelial dysfunction and oxidative stress. Gen Physiol Biophys. 2016; 35:371–7. [PubMed: 27174896]
Social Networks
Human groups form complex social networks of families, communities and nations, and interactions within and between these networks regulate important determinants of health such as health care access, civic policies, economic activity, the structuring of neighborhoods and cities or the generation of environmental pollutants. Consistent with the high impact of social interactions, it has been found that CVD risk factors, such as obesity1 and smoking2, form distinct clusters within social networks, and CVD risk factors, such as obesity, spread through social ties. For instance, it has been reported that a person’s chances of becoming obese increase by 57% if he or she had a friend who became obese within the same period. The strength of social interactions in modifying disease risk seem to exceed the major, known genetic influences. For instance, genome screening has identified that the FTO gene is strongly associated with obesity; however, persons carrying this gene have a 67% increase in the risk of obesity, compared with a 171% increase in risk by having just one friend who is obese,3 suggesting that in this analysis at least, friends have a stronger influence on CVD risk than genetics.
- Christakis NA, Fowler JH. The spread of obesity in a large social network over 32 years. N Engl J Med. 2007; 357:370–9. [PubMed: 17652652]
- Christakis NA, Fowler JH. The collective dynamics of smoking in a large social network. N Engl J Med. 2008; 358:2249–58. [PubMed: 18499567]
- Barabasi AL. Network medicine--from obesity to the "diseasome". N Engl J Med. 2007; 357:404–7. [PubMed: 17652657]
Socioeconomic Status (SES)
Documented effects of SES on CVD also support the role of the environment. Poverty has always been known to be associated with poor health, but perhaps not in the same context as in modern societies. In the early part of the century, CVD was considered to be a disease of affluence, as during the 1930s and 1940s the rates of CVD in the West were higher in men with higher SES,1 but since 1961, CVD mortality have been robustly and negatively associated with SES2. In developing countries; however, CVD rates have increased with increasing affluence and adoption of the Western lifestyle. Reasons for the change in the relationship between SES and CVD remain unclear, but at least in the US, there is consistent evidence that low education is a significant predictor of MI and sudden death. In a remarkable study on 270,000 Bell employees in the US, Hinkle et al. 3, found that men who entered the organization with a college degree had a lower incidence and death rate from CHD in every part of the country and in all departments.
- Antonovsky A. Social class and the major cardiovascular diseases. J Chronic Dis. 1968; 21:65– 106. [PubMed: 5658582]
- Kaplan GA, Keil JE. Socioeconomic factors and cardiovascular disease: a review of the literature. Circulation. 1993; 88:1973–98. [PubMed: 8403348]
- Hinkle LE Jr, Whitney LH, Lehman EW, Dunn J, Benjamin B, King R, Plakun A, Flehinger B. Occupation, education, and coronary heart disease. Risk is influenced more by education and background than by occupational experiences, in the Bell System. Science. 1968; 161:238–46. [PubMed: 5657326]
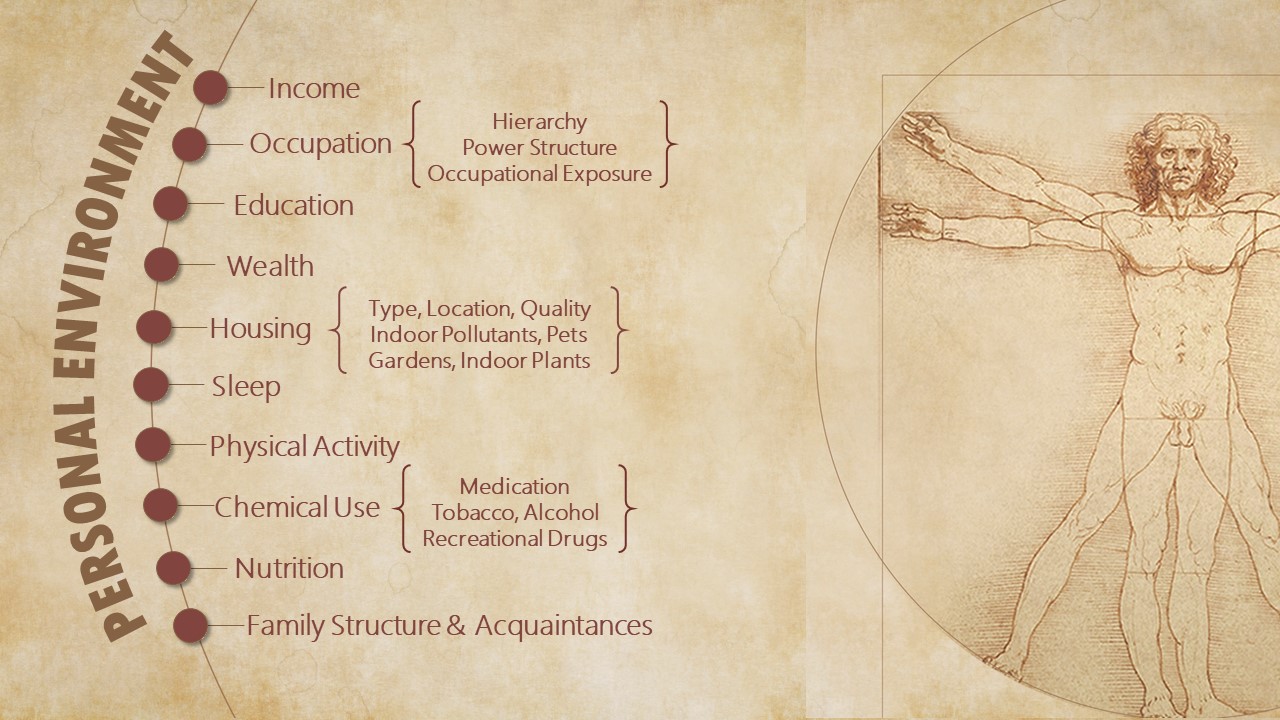
Personal Environment
Personal environments exist within a larger social context. They are shaped by individual circumstance or personal choice. The personal environment of each individual is unique. It is characterized by a person’s occupation, income, family structure, and education. It is populated by lifestyle choices related to nutrition, physical activity, sleep, and the use of recreational drugs, alcohol, and tobacco – choices that are constrained or facilitated by the social environment.
Nutrition
Dietary traditions of obtaining and preparing food are unique to each culture, and like language; they provide cultural identity that is preserved across generations. Individuals from different dietary traditions display relatively stable dietary choices, despite occasional deviations. Dietary choices are also constrained by the social environment and conditions relating to food distribution and availability and often socioeconomic factors, such as affordability. Hence, differences in individual eating behaviors and segregation of heart disease among different communities, families, and socioeconomic strata may be related to culturally transmitted or socially constrained dietary patterns.
Although diet is generally believed to be a critical determinant of health, its effects on CVD risk and progression are difficult to study. For such studies, large populations are required, but they are often heterogeneous in their health characteristics and genetic backgrounds, making it difficult to draw firm conclusions. It is also difficult to vary a single dietary component in one group and not the other, or to maintain large groups of people on specified diets for the long time required to assess changes in CVD risk and progression. Nevertheless, several studies have found significant cardiovascular effects of dietary components. These studies show that changes in diet alone could alter CVD risk. In the Nurses’ Study, replacement of just 5% energy from saturated fat with unsaturated fat was associated with a 42% reduction in CVD risk,1 indicating that independent of energy content, CVD risk is affected by the composition of the diet. This view is reinforced by data on trans fats. In a meta-analysis of 4 studies, a 2% increase in energy consumption from trans fats was found to be associated with a 23% increase in cardiovascular events. A diet high in trans fatty acids has also been associated with 3-fold increase in sudden cardiac death2. Conversely, men who adhered to a “prudent” diet (consisting of vegetables, fruits, whole grains, fish and poultry) have half the CVD risk of men on a “Western” diet (red meat, processed meat, refined grains, sweets and dairy products).3 Similarly, in the PREDIMED trial in which 7447 persons were followed for 4.8 years, a Mediterranean diet supplemented with extra-virgin olive oil or nuts improved the atheroprotective effects of HDL4, delayed atherogenesis5, and reduced the incidence of major cardiovascular events (HR=0.7),6 further underscoring the profound influence of diet on CVD risk and outcomes.
Consumption of saturated fat increases cholesterol levels and high salt intake, the prevalence of hypertension. Trans fats raise LDL cholesterol, but reduce HDL and the size of the LDL particle, making LDL more atherogenic.2 In communities where the consumption of sodium is <1 g/day, the prevalence of hypertension is only 1% of that in industrialized societies, and Tarahumara Indians of Mexico, who consume low (<100 mg/day) levels of cholesterol and fat, have low LDL levels and very low rates of CVD.7 Dietary patterns also affect obesity and T2D, both of which are strongly associated with Western diet,8 saturated fat intake, and frequent consumption of processed meat.9
- Hu FB, Stampfer MJ, Manson JE, Rimm E, Colditz GA, Rosner BA, Hennekens CH, Willett WC. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med. 1997; 337:1491–9. [PubMed: 9366580]
- Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Trans fatty acids and cardiovascular disease. N Engl J Med. 2006; 354:1601–13. [PubMed: 16611951]
- Hu FB, Rimm EB, Stampfer MJ, Ascherio A, Spiegelman D, Willett WC. Prospective study of major dietary patterns and risk of coronary heart disease in men. Am J Clin Nutr. 2000; 72:912– 21. [PubMed: 11010931]
- Hernaez A, Castaner O, Elosua R, Pinto X, Estruch R, Salas-Salvado J, Corella D, Aros F, SerraMajem L, Fiol M, Ortega-Calvo M, Ros E, Martinez-Gonzalez MA, de la Torre R, Lopez-Sabater MC, Fito M. Mediterranean Diet Improves High-Density Lipoprotein Function in HighCardiovascular-Risk Individuals: A Randomized Controlled Trial. Circulation. 2017; 135:633– 643. [PubMed: 28193797]
- Sala-Vila A, Romero-Mamani ES, Gilabert R, Nunez I, de la Torre R, Corella D, Ruiz-Gutierrez V, Lopez-Sabater MC, Pinto X, Rekondo J, Martinez-Gonzalez MA, Estruch R, Ros E. Changes in ultrasound-assessed carotid intima-media thickness and plaque with a Mediterranean diet: a substudy of the PREDIMED trial. Arterioscler Thromb Vasc Biol. 2014; 34:439–45. [PubMed: 24285581]
- Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, Gomez-Gracia E, RuizGutierrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pinto X, Basora J, Munoz MA, Sorli JV, Martinez JA, Martinez-Gonzalez MA. Investigators PS. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013; 368:1279–90. [PubMed: 23432189]
- Connor WE, Cerqueira MT, Connor RW, Wallace RB, Malinow MR, Casdorph HR. The plasma lipids, lipoproteins, and diet of the Tarahumara indians of Mexico. Am J Clin Nutr. 1978; 31:1131–42. [PubMed: 665563]
- van Dam RM, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Dietary patterns and risk for type 2 diabetes mellitus in U.S. men. Ann Intern Med. 2002; 136:201–9. [PubMed: 11827496]
- van Dam RM, Willett WC, Rimm EB, Stampfer MJ, Hu FB. Dietary fat and meat intake in relation to risk of type 2 diabetes in men. Diabetes Care. 2002; 25:417–24. [PubMed: 11874924]
Physical Activity
Physical activity is a central feature of healthy living, and throughout evolution it must have been important for obtaining means for survival (food, material goods, protection, mates, etc) and for avoiding natural or predatory threats. Whatever the reasons, physical inactivity in contemporary humans is strongly associated with CVD risk. It increases the risk of coronary disease by 45%; stroke by 60%; hypertension by 30%; and T2D by 50%, and it has been estimated that 13% of all premature deaths in US could be attributed to physical inactivity.1 Physical inactivity due to prolonged bed rest leads to a decrease in whole body insulin sensitivity within the first 3 days of inactivity,1 and after 12 weeks, to an 8% decrease in LV mass.2 Conversely, regular exercise reduces CVD risk.3 The effects are dose-dependent; moderate physical activity is associated with a 26% reduction in CVD risk, whereas high intensity activities with 42% risk reduction. Moderate to high intensity exercise has been shown to increase life expectancy by 1.3 to 3.7 years and active individuals remain free of CVD 1–3 years longer than their sedentary peers.4
Several mechanisms can account for the salubrious effects of exercise.3 Vigorous physical activity increases myocardial oxygen supply and improves myocardial contraction and electrical stability. In addition, exercise increases HDL levels, while decreasing LDL-C, blood pressure, blood coagulation, systemic inflammation and insulin resistance. Even moderate levels of activity improve lipoprotein profiles and glucose homeostasis. At least some of these effects may be related to an improvement in NO production. Nevertheless, it is unclear how physical activity impacts CVD risk correlates such as cholesterol, blood pressure and insulin sensitivity - which specific metabolic, cellular and metabolic processes are affected and how does physical activity modify the nature and the inter-relationships between different processes that regulate cardiovascular homeostasis and health.
A better understanding of the effects of physical activity is required not only to devise new strategies to promote cardiovascular health and improve athletic performance, but to identify the environmental factors that promote or prevent physical activity. Depression may be one such barrier to physical activity. Individuals who are depressed are physically inactive and therefore the association between depression and CVD could be largely explained by physical inactivity.4 Other barriers to physical activity in modern environments include labor saving devices, efficient transportation, and entertainment modalities that do not require physical activity. Moreover, the built environment, particularly in the US, does not demand, support, or even encourage physical activity. Thus like diet, physical inactivity is not just a lifestyle choice, but a social problem. Therefore, even though personal education and individual motivation remain the bedrock of prevention, community-wide changes in neighborhood characteristics, the built environment, recreational opportunities, and academic curricula are required to create an environment conducive to physical activity.
- Booth FW, Lees SJ. Fundamental questions about genes, inactivity, and chronic diseases. Physiol Genomics. 2007; 28:146–57. [PubMed: 17032813]
- Perhonen MA, Franco F, Lane LD, Buckey JC, Blomqvist CG, Zerwekh JE, Peshock RM, Weatherall PT, Levine BD. Cardiac atrophy after bed rest and spaceflight. J Appl Physiol. 2001; 91:645–53. [PubMed: 11457776]
- Tanasescu M, Leitzmann MF, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Exercise type and intensity in relation to coronary heart disease in men. JAMA. 2002; 288:1994–2000. [PubMed: 12387651]
- Whooley MA, de Jonge P, Vittinghoff E, Otte C, Moos R, Carney RM, Ali S, Dowray S, Na B, Feldman MD, Schiller NB, Browner WS. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008; 300:2379–88. [PubMed: 19033588]
Smoking
No other personal choice has a more negative impact on cardiovascular health than smoking. On average, adults who smoke die 13 to 14 years earlier than non-smokers. In the US, smoking is associated with 443,000 premature deaths per annum, resulting in a yearly loss of over 5 million potential life years and $193 billion in direct medical costs and lost productivity.1 Yet, nearly 16% of Americans continue to smoke, and each day nearly 2,100 youth and young adults become daily smokers (CDC 2016). World-wide, nearly 20% of people smoke. About 5 trillion cigarettes are manufactured each year – nearly 1,000 cigarettes for every person on the planet; 15 billion cigarettes are sold daily, which corresponds to 10 million cigarettes every minute. Although in developed countries, smoking has declined by 40–50% from its peak in the 1960s1, worldwide nearly one billion people continue to smoke.
Although smoking increases the risk of developing lung cancer and respiratory disease, nearly half of the premature mortality associated with smoking is due to CVD. Smokers have a nearly 2-fold higher risk of coronary disease and a 10-fold higher risk of developing peripheral artery disease.2 Smokers are also more susceptible to arrhythmias, stroke and sudden cardiac death.3 Smoking decreases regional left ventricular function even in asymptomatic individuals and significantly (45–80%) increases the risk of heart failure. Compared with non-smokers, even light smokers, who smoke 6–9 cigarettes per day, have a relative MI risk of 2.1.4 Even low level exposure to tobacco smoke can increase CVD risk. This is amply supported by studies showing the high CVD risk of exposure to secondhand smoke5 and the significant decrease in CVD mortality in communities after a smoking ban.6 The high vulnerability of smokers to heart disease underscores the high susceptibility of cardiovascular tissues to inhaled pollutants. Studies on air pollution, secondhand smoke exposure, and smoking have consistently demonstrated cardiovascular injury at levels and durations of exposure much smaller than those associated with lung cancer or even respiratory disease. In addition, exposure to several chemicals including bisphenol A and phthalates,7 persistent organic pollutants8, as well as VOCs such as acrolein, benzene and butadiene9 have been found to be associated with increased CVD risk. Indeed, the WHO estimates that CVD is the leading cause of death due to exposure to environmental pollutants, and the number of CVD deaths far exceed those due to cancer and respiratory disease (Figure 3). Reasons for the high vulnerability of cardiovascular tissue remain unclear, but may relate to poor xenobiotic metabolism in these tissues and their direct exposure to blood borne toxins.
While the mechanisms by which smoking increases CVD risk are not fully understood, it seems to affect CVD independent of other risk factors.10 A meta-analysis of 54 different studies suggests that smoking increases LDL-C and decreases HDL, but changes in lipids account for <10% of the excessive CVD risk in smokers.3 Similarly, even though smoking acutely affects blood pressure, smokers tend to maintain a lower blood pressure and antihypertensive therapy does not completely mitigate the CVD risk of smoking. The contribution of insulin resistance is also uncertain; some studies show that smoking increases insulin resistance but others have found no association between smoking and incident diabetes.11 The mechanisms that mediate the risk of smoking remain to be identified, and even though cardiovascular injury due to tobacco smoke exposure has been directly demonstrated in animal models,3 it is unclear which processes are most vulnerable to exposure and which components of tobacco smoke inflict cardiovascular injury. The problem stems in part from the complexity of the tobacco smoke, which contains more than 4000 different chemicals, making it difficult to attribute cardiovascular injury to specific chemicals, or to generate dose-response relationships. Nevertheless, it is generally believed that the volatile organic compounds (VOCs) generated by combustion contribute to the harmful effects of smoking. Based on this assumption, new devices have been developed that deliver nicotine in an aerosol of propylene glycol and vegetable glycerin. Some investigators believe that these devices – e-cigarettes – are less harmful than combustible cigarettes12 and that they promote cessation,13 a view that is shared by many in the general public. As a result the use of e-cigarette is spreading. In 2014, 3.7% of American adults (9 million people) used e-cigarettes and the use of e-cigarettes among middle and high school students has tripled from 2013 to 2014, accounting for more than 13% of high school students. Currently nearly 2.5 million youth use e-cigarettes (CDC 2014). However, the safety profile of e-cigarettes is unknown.14 While they do not contain many of the harmful or potentially harmful substances generated by combustion, they emit significant levels of reactive aldehydes that have been found to cause cardiovascular toxicity15 as well as PM16 of the same size range that increases CVD risk and mortality.17 Nevertheless, some health advocates find the residual risk associated with e-cigarettes acceptable.18 They believe that e-cigarettes are a safer alternative to combustible cigarettes and their widespread use and acceptance could lessen the burden of tobacco-induced disease. Others, however, are less certain and remain concerned about the potential health effects of nicotine as well as the chemicals present in e-cigarette aerosols.18 They are also concerned about the uptake of e-cigarettes by youth who would not otherwise smoke cigarettes and the renormalization of smoking,14 which has been a major contributing factor to the decline in the number of smokers in the last 5 decades. Regardless, of the impact e-cigarettes might have on cardiovascular health in future, the advent and spread of e-cigarettes is an important case study of how environmental factors – society, culture, advertisement and regulatory policy – influence CVD risk and affect cardiovascular health.
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics-2010 update: a report from the american heart association. Circulation. 2010; 121:e46–e215. [PubMed: 20019324]
- Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004; 43:1731–7. [PubMed: 15145091]
- O'Toole TE, Conklin DJ, Bhatnagar A. Environmental risk factors for heart disease. Rev Environ Health. 2008; 23:167–202. [PubMed: 19119685]
- Schane RE, Ling PM, Glantz SA. Health effects of light and intermittent smoking: a review. Circulation. 2010; 121:1518–22. [PubMed: 20368531]
- Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke. Circulation. 2005; 111:2684– 2698. [PubMed: 15911719]
- Medicine Io. Secondhand Smoke Exposure and Cardiovascular Effects: Making Sense of the Evidence. 2010
- Lind PM, Lind L. Circulating levels of bisphenol A and phthalates are related to carotid atherosclerosis in the elderly. Atherosclerosis. 2011; 218:207–13. [PubMed: 21621210]
- Lind PM, van Bavel B, Salihovic S, Lind L. Circulating levels of persistent organic pollutants (POPs) and carotid atherosclerosis in the elderly. Environ Health Perspect. 2012; 120:38–43. [PubMed: 22222676]
- Bhatnagar A. Cardiovascular pathophysiology of environmental pollutants. Am J Physiol Heart Circ Physiol. 2004; 286:H479–85. [PubMed: 14715496]
- Grundy SM, Balady GJ, Criqui MH, Fletcher G, Greenland P, Hiratzka LF, Houston-Miller N, Kris-Etherton P, Krumholz HM, LaRosa J, Ockene IS, Pearson TA, Reed J, Washington R, Smith SC Jr. Primary prevention of coronary heart disease: guidance from Framingham: a statement for healthcare professionals from the AHA Task Force on Risk Reduction. American Heart Association. Circulation. 1998; 97:1876–87. [PubMed: 9603549]
- Reaven G, Tsao PS. Insulin resistance and compensatory hyperinsulinemia: the key player between cigarette smoking and cardiovascular disease? J Am Coll Cardiol. 2003; 41:1044–7. [PubMed: 12651055]
- Wackowski OA, Bover Manderski MT, Delnevo CD. Comparison of Direct and Indirect Measures of E-cigarette Risk Perceptions. Tob Regul Sci. 2016; 2:38–43. [PubMed: 26855966]
- Etter JF, Bullen C. Electronic cigarette: users profile, utilization, satisfaction and perceived efficacy. Addiction. 2011; 106:2017–28. [PubMed: 21592253]
- Bhatnagar A, Whitsel LP, Ribisl KM, Bullen C, Chaloupka F, Piano MR, Robertson RM, McAuley T, Goff D, Benowitz N. American Heart Association Advocacy Coordinating Committee CoC, Stroke Nursing CoCC, Council on Quality of C and Outcomes R. Electronic cigarettes: a policy statement from the American Heart Association. Circulation. 2014; 130:1418–36. [PubMed: 25156991]
- Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, Prokopowicz A, Jablonska-Czapla M, Rosik-Dulewska C, Havel C, Jacob P 3rd, Benowitz N. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014; 23:133–9. [PubMed: 23467656]
- Soule EK, Maloney SF, Spindle TR, Rudy AK, Hiler MM, Cobb CO. Electronic cigarette use and indoor air quality in a natural setting. Tob Control. 2017; 26:109–112. [PubMed: 26880745]
- Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC Jr, Whitsel L, Kaufman JD. American Heart Association Council on E, Prevention CotKiCD, Council on Nutrition PA and Metabolism. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010; 121:2331–78. [PubMed: 20458016]
- Bhatnagar A. Cardiovascular Perspective of the Promises and Perils of E-Cigarettes. Circ Res. 2016; 118:1872–5. [PubMed: 27283531]
