Current Reseach Projects
After contusive spinal cord injury (SCI), primary loss of neural tissue is followed by secondary damage that exacerbates functional deficits and can be targeted for neuroprotection. Functionally important components of the secondary injury include destruction of axons, death of oligodendrocytes (OLs) and demyelination. The resulting white matter damage is further amplified by neurotoxic inflammation that is mediated by microglia (MG) and monocyte-derived macrophages (MDMs). In addition, spinal cord blood vessels and non-MG glia cells including OLs and astrocytes may contribute to neuroinflammation. After SCI, persistent inflammation may spread to non-injured portions of the central nervous system limiting functional recovery and facilitating secondary complications such as chronic pain and/or depression. Our current projects address role of distinct signaling pathways in SCI-associated acute loss of OLs and post-SCI neuroinflammation. To manipulate those pathways we use genetic and/or pharmacology in the context of the mouse contusive SCI model.
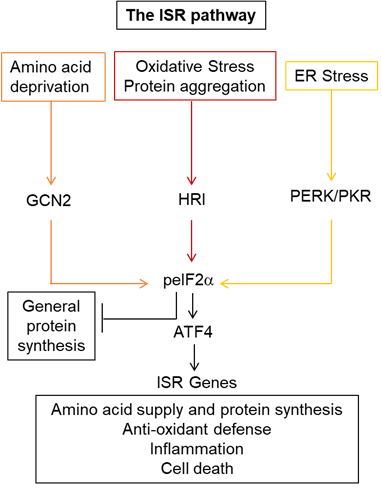
The integrated stress response and oligodendrocyte survival after spinal cord injury (supported by the NINDS award 1R01NS108529, collaboration with dr. Sujata Saraswat-Ohri)Various forms of cell damage/dysfunction activate the integrated stress response (ISR) kinases (PERK, HRI, GCN2, PKR) to phosphorylate the translation factor eIF2α (peIF2α). Inhibition of general protein synthesis and upregulation of the transcription factors ATF4 and CHOP follows. Initially, ISR activation attempts to restore cellular homeostasis. If that fails, prolonged ISR follows and cell death may occur. In addition, HRI-mediated ISR may facilitate inflammation. This project tests the novel hypothesis that after contusive SCI, distinct ISR kinases activate unique cell-specific effector mechanisms with diverse consequences on recovery. Specifically, in OLs, a moderate/transient increase of peIF2α downstream of PERK may restore homeostasis and reduce excessive, pro-apoptotic peIF2α signaling downstream of HRI to promote OL survival and enhance functional recovery. In MG/MDMs, the HRI-peIF2α-ATF4/CHOP pathway enhances cytotoxic neuroinflammation.
|
|---|
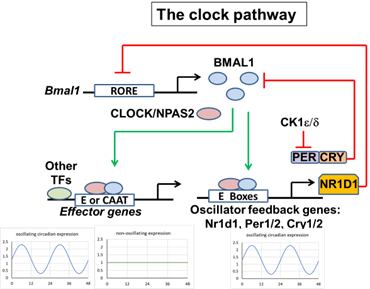
BMAL1/ARNTL plays a critical, non-circadian role in secondary tissue damage after contusive SCI (supported by the NINDS award 1R01NS114404, collaboration with dr. Sujata Saraswat-Ohri)Circadian rhythms regulate a wide spectrum of biological processes of critical importance for organismal health. Mechanistically, circadian rhythms are driven by intrinsic oscillatory changes in gene expression that are orchestrated by several regulators of gene transcription and mRNA translation forming the core oscillator circuitry. BMAL1 is a key, non-redundant component of that pathway. In addition, BMAL1 has also non-circadian functions including anti-oxidant protection in the brain, life span regulation, as well as contributions to atherosclerosis. This project tests a novel hypothesis that BMAL1 regulates OL, endothelial cell, and/or MG/MDM gene expression that contributes to SCI-associated white matter loss, neuroinflammation, and impaired locomotor recovery.
|
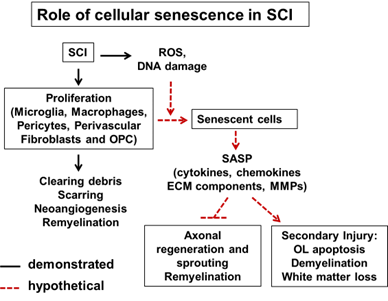
Role of senescent cells in the pathogenesis of contusive spinal cord injury (supported by the NINDS award R21 NS120149)Proliferating cells that accumulate DNA damage may undergo cellular senescence. Senescence-associated induction of the cyclin-dependent kinase inhibitors p21 and p16 triggers permanent exit from the cell cycle. The senescence-associated secretory phenotype (SASP) involves secretion of multiple cytotoxic pro-inflammatory mediators. Elimination of senescent cells (senolysis) improves many age-dependent pathologies and reduces neurodegeneration in mouse models of fronto-temporal dementia or b-amyloidosis. In those cases, improved outcomes were associated with elimination of senescent MGs, astrocytes or OL precursor cells. As those glial cells proliferate in response to CNS injury, their senescence is a likely by-product of trauma-induced gliosis. Therefore, this project tests a hypothesis that accumulation of senescent cells after SCI contributes to progressive white matter loss and reduces recovery of locomotor function.
|