Clinical and Translational Research Resources
Specific Aims
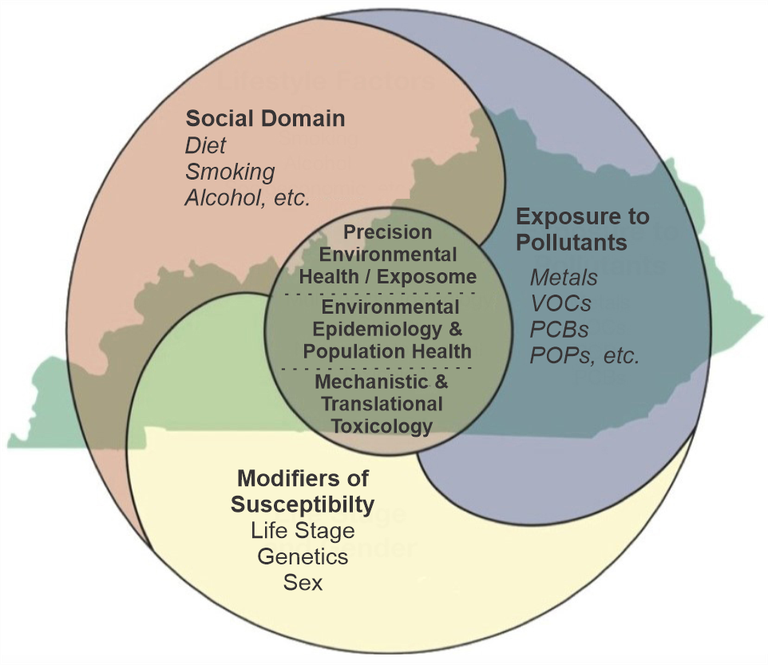
The overarching objective of the Center for Integrated Environmental Health Sciences (CIEHS) is to enhance research on pollution and structural determinants of health influencing human health and chronic diseases, and how life stage and sex modulate the disease response (Fig. 1). The multi-disciplinary Translational Research Support Core (TRSC) provides infrastructure facilitating translational human subjects EHS research for the CIEHS, UofL (through the new Envirome Institute), and the community. The ultimate goal is to improve public health and clinical practice in affected communities. The TRSC supports patient-oriented clinical research (e.g., the early detection, prevention, and therapy of environmentally-related disorders); group and population-based research; as well as community engaged/led research. The TRSC is comprised of two interacting subunits: The Research Administration and Planning Subunit (RAPS) and the Research Implementation Subunit (RIS). Matt Cave, M.D., a physician-scientist and recent NIEHS RIVER awardee, directs the TRSC. The TRSC interacts with other CIEHS Cores to provide access to state-of-the-art instrumentation and technologies; increase multidisciplinary collaboration; and enhance partnerships with community-based organizations (Fig. 2).
The Specific Aims the CIEHS TRSC are:
1. To administer the TRSC and plan translational human subjects EHS research. The RAPS performs these functions. RAPS provides TRSC access to CIEHS members, trainees, and the community. RAPS solicits applications for human subjects research support from CIEHS members, the Administrative Core, the Pilot Project Program, the Research Interest Groups (RIGs), and the community (through the CIEHS Community Engagement Core, CEC). RAPS coordinates the scientific review of research applications and prioritizes projects for support. It applies the NIEHS Translational Research Framework to define the translational nature of each project. Community-proposed research ideas are assigned to an appropriate CIEHS member for project development and execution. RAPS provides translational research training and education. RAPS provides a financial subsidy to TRSC projects offsetting research charges and provides financial oversight to the TRSC. TRSC metrics are tracked and reported to the Administrative Core for program evaluation. RAPS provides scientific, study design, and community engagement consultation through linkages to the RIGs, the Omics and Exposure Assessment Facility Core (OEFC), the Biostatistics and Informatics Core (BIFC), and the CEC. These consultations ensure that all TRSC-supported studies: (i) are rigorously designed and powered; (ii) are consistent with Center’s theme and research interests; and (iii) consider the involvement of community organizations impacting clinical and public health research.
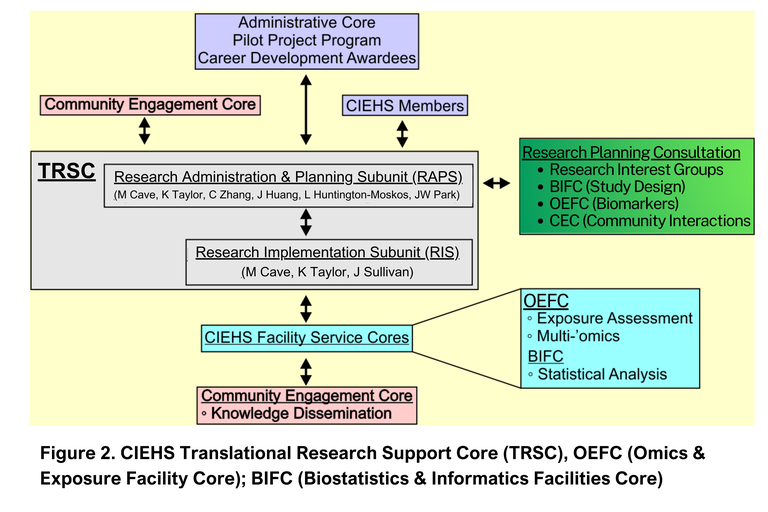
2. To implement TRSC research. The RIS performs this function. RIS provides human subjects research services and access to state-of-the-art bioanalytic instrumentation and technologies through linkage to the Omics & Exposure Assessment Facility Core (OEFC). In turn, the OEFC measures exposure biomarkers (e.g., metals and volatile organic compounds) and ‘omics-based disease biomarkers (e.g., proteomics, metabolomics, transcriptomics, metagenomics, etc.). RIS leverages UofL’s adult Clinical Trials Unit (CTU); the Kosair Charities Pediatric Clinical Research Unit (KCPCRU); as well as UofL’s existing cohorts and biorepositories. CTU/KCPCRU enhance human subjects research through an economy of scale, by providing on-demand fee-for-service access to study personnel, exam rooms, clinical research instruments and ancillary services. Through this innovative approach, RIS provides time and cost-effective support for regulatory compliance; subject recruitment and consent; randomization; retention and follow-up; administration of preventative/therapeutic interventions including study drugs; and data/biological sample collection, processing, storage, retrieval and distribution. Once human data/biological samples are obtained, RIS provides linkage to the OEFC, BIFC, and CEC enabling biomarker measurement, data analysis, and knowledge dissemination to the community, respectively.
Based on the unique combination of physician-scientists who are actively engaged in EHS research and leveraged clinical research units found nowhere else, the CIEHS TRSC has unparalleled capacity to conduct impactful translational EHS research, including biomarker studies and interventional clinical trials.
