Cavity Ring-Down Spectroscopy
Reactive chemical intermediates, especially free radicals, are of crucial importance to combustion and atmospheric chemistry. In our High-Resolution Spectroscopy Lab, we use cavity ring-down (CRD) spectroscopy technique to study the structure and dynamics of free radicals, especially those with zero or low fluorescence quantum yield and hence cannot be detected by laser-induced fluorescence spectroscopy.
CRD is a highly sensitive laser absorption spectroscopy technique that is able to measure absolute absorption cross sections and has found wide applications in molecular spectroscopy. In CRDS, the exponential decay of laser intensity leaking from a high-finesse optical cavity is measured (Figure 1). The time constants of such decay when the cavity is empty and is filled with absorbing species are used to determine the absorption coefficient of the absorber. The absorption coefficient can be determined as:
where c is the speed of light, τ0 is the empty-cavity ring-down time, and τ is the ring-down time for the cavity containing the absorber. With a typical experimental setup (cavity length L=0.5 m, mirror reflectivity R=99.995%), the empty-cavity ring-down time is:
The effective path length is, therefore, τ0c=10 km. Thanks to the long path length, a minimum detectable absorption coefficient of less than one part per million (ppm) per pass can be achieved. The extremely high sensitivity of the CRD technique makes it particularly suitable for investigations of reaction intermediates, which usually are of low concentration.
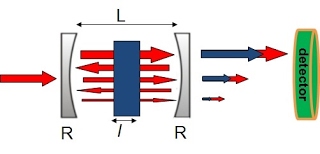
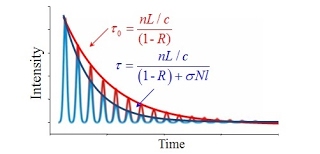
Figure 1: The principle of CRD spectroscopy.
The second major advantage of CRDS is the insensitivity to fluctuations in incident radiation intensity. Unlike in traditional multipass absorption spectroscopy, in which intensity of the transmission is used to determine the absorbance, in CRDS it is determined from the change of the decay constant when the absorbing sample is introduced into the cavity. The intensity of the incident beam, therefore, doesn’t directly affect the absorption cross section measurement.
Furthermore, because most molecules have absorptions due to vibrational motions in the IR region and electronic transitions in the UV/visible region even when the excited state is radiationless or dissociative, absorption-based techniques such as CRD are more versatile than fluorescence-based or photoionization/photoelectron techniques.
2. CRD Spectroscopy Apparatuses
Two CRD spectroscopy apparatuses have been built in our High-Resolution Spectroscopy Lab for detection and investigation of free radicals:
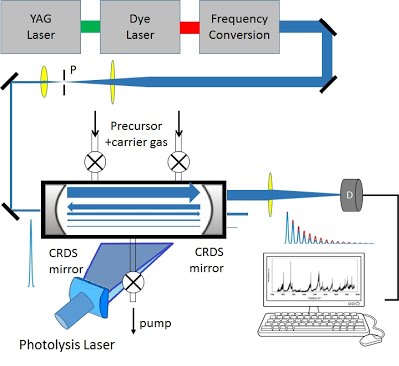
A. Room-Temperature CRD Apparatus using a Flow Cell (Figure 2)
In this experiment, free radicals are produced at room temperature by laser photolysis, often followed by free-radical reactions. All reaction chemistry takes place in the ring-down cell and monitored by delaying the CRD probe pulse from the photolysis pulse on the order of 50-100 μs. Such delay also allows kinetic studies of free radical reactions. To extract the true absorption due to free radicals, the spectrum taken without photolysis, i.e., solely due to the precursors, is subtracted from that with photolysis.
B. Jet-Cooled CRD Apparatus using a Slit-Jet Pulsed Discharge Nozzle (Figure 3).
In the CRDS experiment using a flow cell, radical spectra are usually recorded at near ambient temperature at a total pressure of a few hundred Torr. Many energy levels, therefore, have considerable population according to the Boltzmann distribution, resulting in congested molecular transitions. In addition, the spectral linewidth is large due to Doppler and pressure broadening. Consequentially, the rotational and fine structure of room-temperature spectra and even their vibrational structure in many cases are poorly resolved or not at all. Supersonic jet expansion can drastically cool down the translational, rotational, and vibrational temperatures, simplify molecular spectra, and reduce the spectral linewidth. A pulsed supersonic slit-jet expansion has been combined with a ring-down cavity for the high-sensitivity and high-resolution spectroscopic investigation of free radicals.
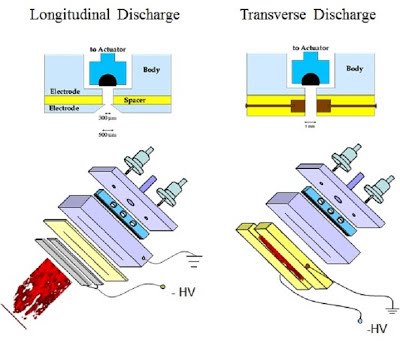
Figure 3: Slit-jet pulsed discharge nozzle for jet-cooled CRD spectroscopy.
Two configurations of pulsed discharge are illustrated.
C. Lasers
The existing laser systems in our lab can cover mid-IR, near-IR, visible, and UV ranges almost continuously. An output from a tunable pulsed dye laser pumped by a Q-switched Nd: YAG laser and its frequency doubling can be employed for CRD measurements in the visible and the UV regions (200-900 nm). The near-IR region (900-1500 nm) can be covered using H2 Raman shift cells (single-pass or multi-pass). A difference frequency mixer that combines the outputs from the dye laser and the fundamental output (1024 nm) of the Nd: YAG laser extends the wavelength range to mid-IR (1.8 to 4.2 um). In the room-temperature CRD experiment either an excimer lasers or the third (355 nm) or fourth (266 nm) harmonics of a Nd: YAG laser is used for photolysis.
D. Production of free radicals radicals
In our room-temperature CRD spectroscopy investigations, free radicals are usually produced by UV laser photolysis of judiciously selected precursors, often followed by free-radical reactions. Generating free radicals under jet-cooled conditions is more challenging because producing free radicals by photolysis is not applicable in this case: The pulse duration of the photolysis lasers, either Nd: YAG lasers or excimer lasers, is about 10 ns, while the photolysis beam needs to be focused along the downstream direction to a couple of millimeters. The transit time, viz, the time for the produced free radicals to interact with the CRD laser, is therefore limited to a couple of microseconds, much lower than the ring-down time. In a supersonic jet expansion, free radicals are usually generated using discharge or pyrolysis.


