Research
|
Transient-absorption spectroscopy of solar cell materials |
|---|
|
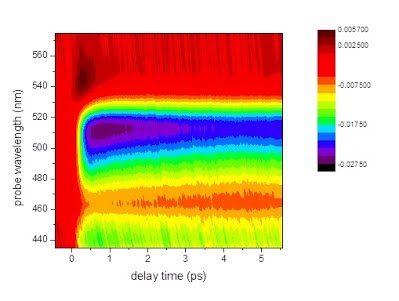
A transient absorption spectrum of the CdSe quantum dots coated with HPTC ligands. |
|
The efficiency of solar cell panels is determined by the photoinduced dynamics. For example, in organic solar cells, the optical absorption of the electron “donor” and the injection of electrons from the “donor” into the “acceptor”, which generate the charge separation, must be accelerated and the back electron transfer and recombination, which destroy the charge separation, must be suppressed. Ultrafast laser spectroscopy provides a powerful tool to quantitatively measure and hence understand these procedures, which can help to improve the conversion efficiency, and even invent new types of solar cells. In our ultrafast spectroscopy lab, an experimental system for transient absorption spectroscopy has been set up to study the dynamics that contribute to the energy transfer and charge separation in solar cells. A laser pulse with ultrashort time duration (~30 fs) is used to pump the system, while a second ultrashort laser pulse with either a continuous wavelength distribution (so-called “white light”) or with a narrow wavelength range is used to “probe” the photoinduced dynamics. Our investigations include transient absorption spectroscopy of both dye-sensitized solar cells based on nanoparticles and nanowires, and organic solar cells. |
| Laser-induced fluorescence/dispersed fluorescence (LIF/DF) spectroscopy |
|---|
|
|
|
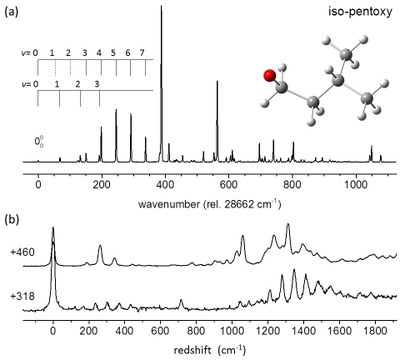
An apparatus for laser-induced fluorescence/dispersed fluorescence (LIF/DF) spectroscopy of reaction intermediates under supersonic-jet expansion conditions has been built. LIF spectra map out the excited-state vibronic structure of the target molecules, while DF spectra reveal the ground-state vibrational energy levels. Free radicals are generated using either laser photolysis or pulsed electric discharge of appropriate precursors. |
| Cavity ring-down (CRD) spectroscopy |
|---|
|
|
|
CRD is a highly sensitive laser absorption spectroscopy technique that is able to measure absolute absorption cross sections. Two CRD spectroscopy apparatuses have been built in our Lab for detection and investigation of free radicals: room-temperature CRD apparatus using a flow cell and jet-cooled CRD apparatus using a slit-jet pulsed discharge nozzle. |
| High-resolution spectroscopy of free radicals |
|---|
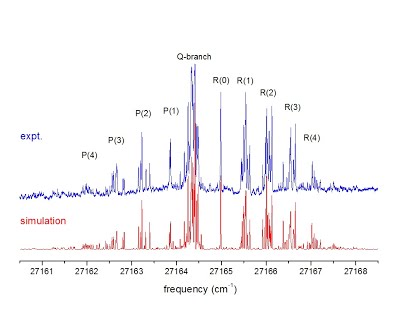
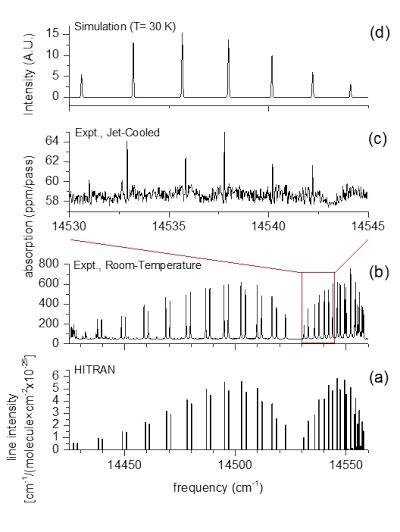
Experimental (top) and simulated (bottom) spectra of the isopropoxy radical compared in the SpecView program. |
|
“Free radicals”, molecules and molecular ions with one unpaired electron, are highly chemically reactive and play an important role in combustion, atmospheric chemistry, polymerization, plasma chemistry, biochemistry, and many other chemical processes. Chemical reaction intermediates are often free radicals. Study of free radicals, therefore, is critical to chemical kinetics. Molecular spectroscopy, especially high-resolution spectroscopy, is a powerful tool to detect and investigate free radicals. By simulating and fitting the experimentally observed spectra of free radicals, one can determine their structures and geometries, identify different isomers and conformers, and unravel the complicated intramolecular interaction among the electronic motion and the vibration and rotation of the nuclei. The figure left demonstrates the rotationally resolved laser-induced fluorescence spectrum of the isopropoxy and the simulation using a novel coupled two-state spectroscopic model. |
| The Jahn-Teller and Pseudo-Jahn-Teller Effects |
|---|
|
|
|
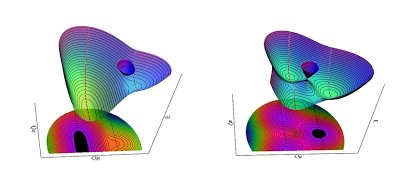
The coupling between the motion of electrons and vibration of nuclei in a polyatomic molecule in an orbitally degenerate state leads to spontaneous geometrical distortion and removal of molecular symmetry. The two otherwise degenerate electronic states now meet at a singularity and form a "conical intersection". This so-called "Jahn-Teller interaction" occurs in both isolated molecules and crystal lattice and leads to many interesting physical phenomena including the molecular Aharonov-Bohm effect and superconductivity. Previously, the result of spectroscopic analysis on a prototypical Jahn-Teller molecule, the methoxy radical (CH3O), has enabled the determination of geometric change as small as ~1 mÅ in bond lengths and ~1 degree in bond angles. Recently, the study of the methoxy radical has been extended to its asymmetrically deuterated isotopologues (CH2DO and CHD2O) and to larger alkoxy radicals such as ethoxy, isopropoxy, and cyclohexyl which exhibit the so-called "pseudo-Jahn-Teller effect". (See the molecular structures and potential energy surfaces of ethoxy and isopropoxy left.) The theoretical, computational and spectroscopic studies on the Jahn-Teller and pseudo-Jahn-Teller molecules will provide quantitative insight into the dynamics around conical intersections. Conical intersections are ubiquitous in molecules and play a crucial role in chemistry as they act as conduits to convert electronic energy into nuclear kinetic energy.Potential energy surfaces of ethoxy (left) and isopropoxy (right) demonstrating the pseudo-Jahn-Teller effect. |