Earth-abundant Electrocatalysts
Catalysts are a critical component in solar fuels to minimize the activation barrier that must be overcome in the electrochemical reactions. Many state-of-the-art electrocatalysts are based on rare noble metals that may not be manufactured cost-effectively for the large-scale energy industry. Replacing these rare materials with earth-abundant elements while maintaining high activity is a challenge.
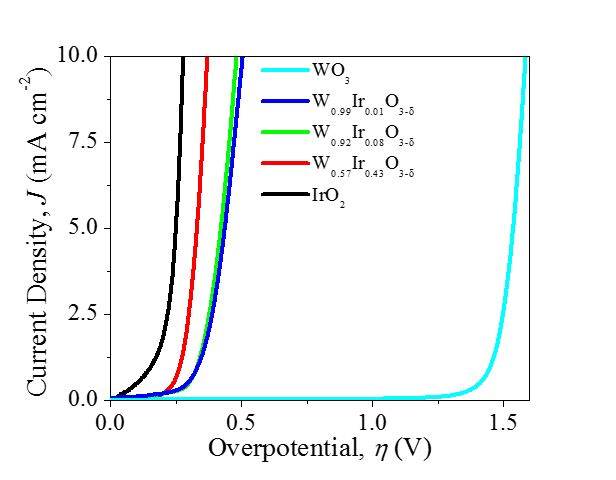 For electrochemical fuel synthesis reactions, active catalysts are needed for both the reduction and oxidation half-reactions at the cathode and anode, respectively, to minimize energy losses to reaction kinetics. There are many advantages for commercial electrolyzers to operate in acidic media, but one limitation is the harsh corrosive conditions at the anode. Only the rare material IrO2 and select other noble metal catalysts have proven effective under these conditions. Conn Center research is currently focused on developing alternative low-noble-metal electrocatalysts for water oxidation with acid-stability and high activity. A novel plasma synthesis technique is being employed to make metastable compositions of mixed metal oxides with distinct properties from oxides produced through traditional thermal oxidation.
For electrochemical fuel synthesis reactions, active catalysts are needed for both the reduction and oxidation half-reactions at the cathode and anode, respectively, to minimize energy losses to reaction kinetics. There are many advantages for commercial electrolyzers to operate in acidic media, but one limitation is the harsh corrosive conditions at the anode. Only the rare material IrO2 and select other noble metal catalysts have proven effective under these conditions. Conn Center research is currently focused on developing alternative low-noble-metal electrocatalysts for water oxidation with acid-stability and high activity. A novel plasma synthesis technique is being employed to make metastable compositions of mixed metal oxides with distinct properties from oxides produced through traditional thermal oxidation.

