Jason Chesney, M.D., Ph.D.
Education: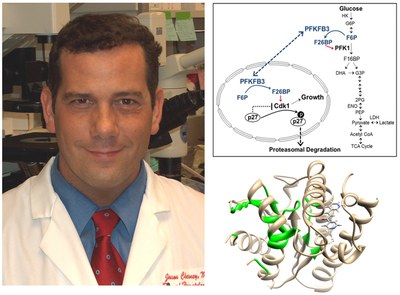
B.A., Anthropology, Summa Cum Laude, University of Minnnesota, 1987-1991
Ph.D., Biomedical Sciences, University of Minnesota Graduate School, 1993-1997
M.D., University of Minnesota Medical School, 1991-1998
Internship and Residency, Cornell University Medical College, 1998-2001
Residency, Memorial Sloan Kettering Cancer Center, 1998-2001
Board Certified, American Board of Internal Medicine, 2001-2011
Clinical Fellowship, Immunology, Cornell University Medical College, 2001-2002
Post-Graduate Clinical Training, Medical Oncology/Hematology, University of Louisville, 2003-2005
Curriculum Vitae
Current Positions:
Director, J. Graham Brown Cancer Center, University of Louisville
James Graham Brown Foundation Endowed Chair, Brown Cancer Center
Professor, Depts. of Medicine (Hematology/Oncology), Biochemistry/Molecular Biology and Pharmacology/Toxicology
Director, J. Graham Brown Cancer Center Clinical Research Program and Biorepository
Chairman, Medical Oncology and Hematology Data and Safety Monitoring Committee
Contact Information:
Research Description
Dr. Chesney's laboratory discovered that a regulator of glucose metabolism, 6-phosphofructo-2-kinase (PFKFB3), is over-expressed in leukemia and adenocarcinoma cells (1), required for de novo nucleic acid synthesis (1), increased in human solid tumors relative to adjacent normal tissues (2), and essential for Ras transformation and tumor growth in vivo (3), the activation of cyclin-dependent kinases (4, 5) and the estrogen-mediated survival of ER+ breast cancer cells (6). Working with Peter Carmeliet at the Katholieke Universiteit, his group recently observed that PFKFB3 also is required for angiogenesis and vessel sprouting (7, 8). In 2008, Dr. Chesney reported with Dr. John Trent the discovery of the first PFKFB3 small molecule antagonist, 3PO – that this compound acutely suppressed the glucose metabolism of cancer cells but not normal epithelial cells and suppressed tumor growth in mice (9). Based on these studies, they developed a collaboration with an industry partner, Advanced Cancer Therapeutics, to screen synthetic derivatives of the parent compound (10). This effort led to the identification of a synthetic derivative of 3PO termed PFK158 that is currently undergoing evaluation by Dr. Rebecca Redman in a phase I trial (Phase 1 Safety Study of ACT-PFK-158, 2HCl in Patients With Advanced Solid Malignancies, clinicaltrials.gov #NCT02044861). They are now testing the ability of PFK-158 to overcome the intrinsic and acquired resistance of cancer cells to multiple targeted, immunotherapeutic and radiation therapies including BRAF inhibitors for melanoma patients (vemurafenib), EGFR inhibitors for lung cancer patients (erlotinib), anti-estrogen agents for breast cancer patients (fulvestrant) and anti-CTLA4 antibodies for all types of cancer (ipilimumab).
Dr. Chesney's group has expanded their original studies on PFKFB3 to additional metabolic pathways and molecular targets in cancer. For example, their research group has established that the oncogene Ras activates glycolytic flux into the TCA cycle in immortalized bronchial epithelial cells (11) and identified additional enzymes that are regulated by oncogenes and essential for activating aberrant metabolic pathways required for transformation including aspartate aminotransferase (12), choline kinase (13) and cytochrome c oxidase (14). Importantly, they recently synthesized a novel choline kinase inhibitor that has anti-tumor activity without significant toxicity (15) and are currently optimizing this small molecule to improve potency and pharmacokinetic properties.
Dr. Chesney has been funded by the National Cancer Institute, the Congressionally Directed Medical Research Program and the National Center for Research Resources/National Institute of General Medical Sciences as well as multiple pharmaceutical sponsors. He serves as a reviewer on multiple National Cancer Institute subcommittees to evaluate Specialized Programs of Research Excellence (SPOREs), Tumor Cell Biology and P30-Designated Cancer Center grants. He has been named a Top Doctor by U.S. News and World Report for Solid Tumors and Clinical Trials and is the recipient of the Kentucky Derby Julep Scientist of the Year Award.
Learn about Dr. Chesney's clinical research programs.
Representative Publications:
- Chesney J, Mitchell R, Benigni F, Bacher M, Spiegel L, Al-Abed Y, Han JH, Metz C, Bucala R. An inducible gene for 6-phosphofructo-2-kinase (iPFK-2) with an AU-rich mRNA instability element: Role in tumor cell glycolysis and the Warburg Effect. Proceedings of the National Academy of Sciences USA 1999;96(6):3047-52. PMID: 10077634.
- Atsumi T*, Chesney J*, Metz C, Leng L, Donnelly S, Makita Z, Mitchell R, Bucala R. High expression of inducible 6-phosphofructo-2-kinase (iPFK-2; PFKFB3) in human cancers. Cancer Research 2002;62(20):5881-7. (*authors contributed equally) PMID: 12384552.
- Telang S, Yalcin A, Clem AL, Bucala R, Lane AN, Eaton JW, Chesney J. Ras transformation requires metabolic control by 6-phosphofructo-2-kinase. Oncogene 2006;25(55):7225-34. PMID: 16715124.
- Yalcin A, Clem BF, Simmons A, Lane A, Nelson K, Clem A, Brock E, Siow D, Wattenburg B, Telang S, Chesney J. Nuclear targeting of 6-phosphofructo-2-kinase (PFKFB3) increases proliferation via cyclin-dependent kinases. Journal of Biological Chemistry 2009;284(36): 24223-32. PMID: 19473963.
- Yalcin A, Clem BF, Imbert-Fernandez Y, Ozcan SC, Peker S, O’Neal J, Klarer AC, Clem AL, Telang S, Chesney J. 6-Phosphofructo-2-kinase (PFKFB3) promotes cell cycle progression and suppresses apoptosis via Cdk1-mediated phosphorylation of p27. Cell Death & Disease (Nature) 2014;5:e1337. PMID: 25032860; PMCID: PMC4123086.
- Imbert-Fernandez Y, Spaulding R, Lanceta L, Clem BF, O’Neal J, Clem A, Telang S, Chesney J. Estradiol stimulates glucose metabolism via 6-phosphofructo-2-kinase (PFKFB3). Journal of Biological Chemistry 2014;289(13):9440-8. PMID: 24515104; PMCID: PMC3979387.
- De Bock K, Georgiadou M, Schoors S, Cauwenberghs S, Cascante M, Telang S, De Berardinis R, Schoonjans L, Vinckier S, Chesney J, Ghesquière B, Gerhardt H, Dewerchin M, Carmeliet P. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 2013;154:651-63. PMID: 23911327.
- Schoors S, De Bock K, Cantelmo AR, Georgiadou M, Ghesquière B, Cauwenberghs S, Kuchnio A, Wong BW, Quaegebeur A, Goveia J, Bifari F, Wang X, Blanco R, Tembuyser B, Cornelissen I, Bouché A, Vinckier S, Diaz-Moralli S, Gerhardt H, Telang S, Cascante M, Chesney J, Dewerchin M, Carmeliet P. Partial and transient reduction of glycolysis by PFKFB3 blockade reduces pathological angiogenesis. Cell Metabolism 2014;19(1):37-48. PMID: 24332967.
- Clem B, Telang S, Clem A, Yalcin A, Meier J, Simmons A, Rasku M, Dean W, Trent JO, Chesney J. Small molecule inhibition of 6-phosphofructo-2-kinase activity suppresses established tumor growth. Molecular Cancer Therapeutics 2008;7(1):110-20. PMID: 18202014.
- Clem BF, O’Neal J, Tapolsky G, Clem A, Imbert-Fernandez Y, Klarer AC, Redman R, Trent JO, Telang S, Chesney J. Targeting 6-phosphofructo-2-kinase (PFKFB3) as a therapeutic strategy against cancer. Molecular Cancer Therapeutics (AACR) 2013;12(8):1-10. PMID: 23674815. Selected for cover page of August 2013 Molecular Cancer Therapeutics Issue.
- Telang S, Nelson K, Lane A, Chesney J. The oncoprotein H-RasV12 increases mitochondrial metabolism. Molecular Cancer 2007;6(1):77-83. PMID: 17598900.
- Thornburg J, Nelson K, Clem BF, Lane A, Arumugam S, Simmons A, Eaton JW, Telang S, Chesney J. Targeting aspartate aminotransferase in breast cancer. Breast Cancer Research 2008;10:R84. PMID: 18922152.
- Yalcin A Clem BF, Makoni S, Clem A, Nelson K, Thornburg J, Siow D, Lane AN, Brock SE, Goswami U, Eaton JW, Telang S, Chesney J. Selective inhibition of choline kinase simultaneously attenuates MAPK and PI3K/AKT signaling. Oncogene 2010;29(1):139-49. PMID: 19855431.
- Telang S, Nelson KK, Siow DL, Yalcin A, Thornburg JM, Imbert-Fernandez Y, Klarer AC, Farghaly H, Clem BF, Eaton JW, Chesney J. Cytochrome c oxidase is activated by the oncoprotein Ras and is required for A549 lung adenocarcinoma growth. Molecular Cancer 2012;11(1):60-72. PMID: 22917272.
- Clem B, Clem A, Yalcin A, Goswami U, Arumugam S, Telang S, Trent J, Chesney J. A novel small molecule antagonist of choline kinase-a that simultaneously suppresses MAPK and PI3K/AKT signaling. Oncogene 2011;30(30):3370-80. PMID: 21423211.
- Klarer AC, O'Neal J, Imbert-Fernandez Y, Clem A, Ellis SR, Clark J, Clem B, Chesney J, Telang S. Inhibition of 6-phosphofructo-2-kinase (PFKFB3) induces autophagy as a survival mechanism. Cancer Metabolism 2014 Jan 23;2(1):2. doi: 10.1186/2049-3002-2-2. PMID: 24451478; PMCID: PMC3913946.
- Chesney J, Clark J, Klarer AC, Imbert-Fernandez Y, Lane AN, Telang S. Fructose-2,6- bisphosphofructo-2-kinase/fructose-2,6-bisphosphatase 4 (PFKFB4) is required for the glycolytic response to hypoxia and tumor growth. Oncotarget 2014 Aug 30;5(16):6670-86. PMID: 25115398; PMCID: PMC4196155.
- Liu Y, Lu X, Huang L, Wang W, Jiang G, Dean KC, Clem B, Telang S, Jenson AB, Cuatrecasas M, Chesney J, Darling DS, Postigo A, Dean DC. Different thresholds of ZEB1 are required for Ras-mediated tumour initiation and metastasis. Nature Communications 2014 Dec 1;5:5660. doi: 10.1038/ncomms6660. Erratum in: Nat Commun. 2015;6:6699. PMID: 25434817.
- Chesney J, Clark J, Lanceta L, Trent JO, Telang S. Targeting the sugar metabolism of tumors with a first-in-class 6-phosphofructo-2-kinase (PFKFB4) inhibitor. Oncotarget 2015 Jul 20;6(20):18001-11. PMID: 26221874; PMCID: PMC4627231.
- Clem BF, O'Neal J, Klarer AC, Telang S, Chesney J. Clinical development of cancer therapeutics that target metabolism. QJM 2016 Jun;109(6):367-72. Review. PMID: 26428335; PMCID: PMC4900488.
- O'Neal J, Clem A, Reynolds L, Dougherty S, Imbert-Fernandez Y, Telang S, Chesney J, Clem BF. Inhibition of 6-phosphofructo-2-kinase (PFKFB3) suppresses glucose metabolism and the growth of HER2+ breast cancer. Breast Cancer Research & Treatment 2016 Nov;160(1):29-40. PMID: 27613609.
- Chesney JA, Mitchell RA, Yaddanapudi K. Myeloid-derived suppressor cells-a new therapeutic target to overcome resistance to cancer immunotherapy. Journal of Leukocyte Biology 2017 Sep;102(3):727-40. doi: 10.1189/jlb.5VMR1116-458RRR. Review. PMID: 28546500.
- Rodig SJ, Gusenleitner D, Jackson DG, Gjini E, Giobbie-Hurder A, Jin C, Chang H, Lovitch SB, Horak C, Weber JS, Weirather JL, Wolchok JD, Postow MA, Pavlick AC, Chesney J, Hodi FS. MHC proteins confer differential sensitivity to CTLA-4 and PD-1 blockade in untreated metastatic melanoma. Science Translational Medicine 2018 Jul 18;10(450). pii: eaar3342. doi: 10.1126/scitranslmed.aar3342. PMID: 30021886.
- Mondal S, Roy D, Sarkar Bhattacharya S, Jin L, Jung D, Zhang S, Kalogera E, Staub J, Wang Y, Xuyang W, Khurana A, Chien J, Telang S, Chesney J, Tapolsky G, Petras D, Shridhar V. Therapeutic targeting of PFKFB3 with a novel glycolytic inhibitor PFK158 promotes lipophagy and chemosensitivity in gynecologic cancers. International Journal of Cancer 2019 Jan 1;144(1):178-89. doi: 10.1002/ijc.31868. Epub 2018 Oct 30. PMID: 30226266; PMCID: PMC6261695.
- Chesney J, Imbert-Fernandez Y, Telang S, Baum M, Ranjan S, Fraig M, Batty N. Potential clinical and immunotherapeutic utility of talimogene laherparepvec for patients with melanoma after disease progression on immune checkpoint inhibitors and BRAF inhibitors. Melanoma Research 2018 Jun;28(3):250-255. doi: 10.1097/CMR.0000000000000444. PMID: 29561296. PMCID: PMC5929488.
- Rodig SJ, Gusenleitner D, Jackson DG, Gjini E, Giobbie-Hurder A, Jin C, Chang H, Lovitch SB, Horak C, Weber JS, Weirather JL, Wolchok JD, Postow MA, Pavlick AC, Chesney J, Hodi FS. MHC proteins confer differential sensitivity to CTLA-4 and PD-1 blockade in untreated metastatic melanoma. ScienceTranslationalMedicine 2018 Jul 18;10(450):eaar3342. doi: 10.1126/scitranslmed.aar3342. PMID: 30021886.
- Lypova N, Telang S, Chesney J, Imbert-Fernandez Y. Increased 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 activity in response to EGFR signaling contributes to non-small cell lung cancer cell survival. Journal of Biological Chemistry 2019 Jul 5;294(27):10530-10543. doi: 10.1074/jbc.RA119.007784. Epub 2019 May 24. PMID: 31126985. PMCID: PMC6615683.
- Chesney J, Puzanov I, Collichio F, Milhem MM, Hauschild A, Chen L, Sharma A, Garbe C, Singh P, Mehnert JM. Patterns of response with talimogene laherparepvec in combination with ipilimumab or ipilimumab alone in metastatic unresectable melanoma. BritishJournal ofCancer 2019 Aug;121(5):417-420. doi: 10.1038/s41416-019-0530-6. Epub 2019 Jul 29. PMID: 31353364. PMCID: PMC6738060.
- Ozcan SC, Sarioglu A, Altunok TH, Akkoc A, Guzel S, Guler S, Imbert-Fernandez Y, Muchut RJ, Iglesias AA, Gurpinar Y, Clem AL, Chesney JA, Yalcin A. PFKFB2 regulates glycolysis and proliferation in pancreatic cancer cells. Molecular&CellularBiochemistry 2020 Jul;470(1-2):115-129. doi: 10.1007/s11010-020-03751-5. Epub 2020 May 15. PMID: 32415418.
- Lanceta L, Lypova N, O'Neill C, Li X, Rouchka E,Chesney J, Imbert-Fernandez Y. Differential gene expression analysis of palbociclib-resistant TNBC via RNA-seq. Breast Cancer Research & Treatment 2021 Apr;186(3):677-686. doi: 10.1007/s10549-021-06127-5. Epub 2021 Feb 18. PMID: 33599863. PMCID: PMC8019424.
 Facebook
Facebook Twitter
Twitter Linkedin
Linkedin