Research Billing
Institutional Research Billing Compliance (RBC) Review
In order to comply with federal, state and institutional regulations, the University is responsible for establishing effective processes to ensure that all services for a clinical research study are billed properly. This is a complex process because clinical trials often involve multiple entities. A research study visit may have routine medical care in addition to services or procedures conducted for research purposes. Routine Care is defined as the procedures/services that are medically necessary and would have been provided to the research subject were they not participating in the clinical research.
A Medicare coverage analysis (MCA) is required for all clinical research projects in which tests, procedures, and interventions associated with them are invoiced to third party payers. MCA identifies the services for which Medicare will pay under the Medicare Clinical Trial Policy. A methodical analysis helps avoid compliance pitfalls with regard to inappropriate billing. Non-compliant billing is subject to severe penalties, as well as civil and criminal actions.
UofL’s institutional research billing compliance review is a seamless step in the project start-up process. The only additional component is that an institutional approval letter needs to be issued prior to finalizing your budget with the Sponsor. The clinical trial agreement will not be finalized without this approval letter.
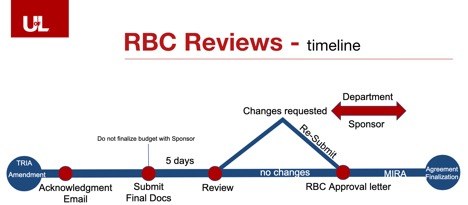
For more detailed information, please click here to download the slide deck.
For all questions related to compliance reviews, please send an e-mail to ccdfin@louisville.edu
